Abstract
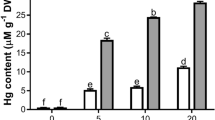
Thirty-day-old seedlings of tomato (Lycopersicon esculentum Mill.) were treated with various Hg concentrations (0, 10, and 50 μM) for up to 20 days, and the hypothesis that Hg induces oxidative stress leading to the reduction of biomass and chlorophyll content in leaves was examined. The accumulation of Hg in seedlings increased with external Hg concentration and exposure time, and Hg content in roots exposed to 50 μM Hg for 20 days was about 27-fold higher than that in shoots. Furthermore, Hg exposure not only reduced biomass and chlorophyll levels in leaves but also caused an overall increase of endogenous H2O2, lipid peroxidation products (malondialdehyde), and antioxidant emzymes activities such as superoxide dismutase, catalase, and peroxidase in leaves and roots. Our results suggest that the suppression of growth and the reduction of chlorophyll levels in tomato seedlings exposed to toxic Hg levels may be caused by an enhanced production of active oxygen species and subsequent high lipid peroxidation.
Similar content being viewed by others
Literature Cited
Anion Dl (1949) Copper enzymes in isolated chloro-plasts. Polyphenol oxidase inBeta vulgaris. Plant Physiol24: 1–15
Asada K (1996) Radical production and scavenging in the chloroplasts.In AB Baker, ed, Photosynthesis and the Environment, Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 123–150
Becana N, Aparcio-Tejo P, Irigoyen JJ, Sanchez-Diaz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and rool nodules ofMedicago sativa. Plant Physiol82: 1169–1171
Beers RF, Sizer IW (1952) A spec tropholometrk melhocl lor measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem195:133–140
Burzynski M (1987) The influence of lead and c alcium on the absorption and distribution of potassium, (allium, magnesium and iron in cucumber seedlings. Ada Physiol plant9: 229–238
Cataldo DA, Garland TR, Wildung RE (l981) Cadmium distribution and chemical fate in soybean plants. Plant Physiol68: 835–839
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Fnzymol2: 764–775
Chaoui A, Mazhoudi S, Ghorbal MH, Ferjani FE (1997) Cadmium and zinc induucation of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.) Plant Science127: 139–147
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O, and catalase during thermo-tolerance induced by salicylic acid or heal aclimation in mustard seedlings. Plant Physiol116: 1351–1357
De Vos CHR, Ten Boukum WM, Vooijs R, Schat H, De Kok LJ (1993). Effect of J opper on Tally and composi-tion and peroxidation of lipids in the roots ol copper-tolerant and -sensitiveSilene cucubalus. Plain hysiol Biochem31: 151–158
del Rio LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jimenez A, Lopez-Huertas E, Hernandez JA (1998) The activated oxygen role of peroxisomes in senescence. Plant Physiol116: 1195–1200
Dhindsa RS, Dhindsa P, Thorpe TA (1987) Leaf senescence correlated with increased levels ol membiane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot32: 93–101
Elstner EF, Wagner GA, Schultz W (1988) Ac livatecl oxygen in green plants in relation to stress situations. Curr. Top Plant Biochem Physiol7: 159–187
Foyer CH, Lelandais M, Kunert KJ (1994) Pholooxidative stress, in plants. Physiol Plant92: 696–7l7
Fridovich I (1986) Biological effects ol the superoxide radical. Arch Biochem Biophys24: 1–11
Gupta SC, Goldsbrough PB (1991) Phvtochelalin accumulation and cadmium tolerance in selected tomato cell lines. Plant Physiol97: 306–312
Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and discvise. Biochem J219: 1–14
Huang YL, Cheng SL, Lin TH (199b) Lipid peroxidation in rats administered with mercuric chloride. Biol Trace Flem Res52: 193–206
Kang KS, Lim CJ, Han TJ, Kim JC, Jin CD (1998) Ac tiva-tion ol ascorbate-glutalhione cycle inArabiclopsis leaves in response to aminotriazole. J Plant Biol41: 155–161
Koricheva J, Roy A, Vranjic JA, Haukioja E, Hughes PR, Hanninen O (1997) Antioxidant responses to simulated acid ram and heavy metai deposition in bitch seedlings. Environ Pollut95: 249–258
Lozano-Rodriguez E, Hernandez Lt, Bonay P, Carpena-Ruiz RO (1997) Distribution ol cadmium in shoot and root tissues of maize and pea plants. Physiological disturbances. J Exp Bot306: 123–128
Luna CM, Gonzalez CA, Trippi VS (1994). Oxidative damage caused by an excess of copper in oat leaves. Plant Cell Phvsiol35: 11–15
Mazhoudi S, Chaoui A, Ghorbal MH, Ferjani EE (1997). Response ol antioxidant enzymes to excess copper in tomato (Lycopersion esculrnturn Mill). Plant Science127: 129–137
Neumann D, Nieden UZ, Schwieger W, Leopold I, Lich-tenberger O (1997) Heavy metal tolerance of Minuar-tia vema. J Plant Physiol151: 101–108
Ouariti O, Boussama N, Zarrouk M, Cherif A, Ghorbal MH (1997) Cadmium-and copper-induced changes in tomato membrane lipids. Phyluc hemistry45:1343–1350
Patterson BD, McRae BA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using tita-nium(IV). Anal Bioc hem139: 487–492
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-indue ed oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell6: 65–74
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of radmium mobility and accumulation in Indian mustard. Plant Physiol109: 1427–1433
Sanchez M, Revilla G, Zarra I (1995) Changes in peroxidase activity associated with cell walls during pine hypocotyl growth. Ann Bot75: 415–419
Scandalios JG (1993) Oxygen stress and superoxide dismisses. Plant Physiol101: 7-l2
Sinha S, Gupta M, Chandra P (1997) Oxidative stress induced bv iron inHydatid verticillata (i.f.) Royle: response of antioxidants. Ecolox and Fnviron Safety38: 286–291
Somashekaraiah BV, Padmaja K, Prasad ARK (1992) Phylotoxicily of cadmium ions on germinating seedlings of mung bean (Phaseolus valgaris): involvement of lipid peroxides in chlorophyll degradation. Physiol Plant85: 85–89
Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant cell Fnviron13: 195–206
Van Assche F, Put C, Clijsters H (1986) Heavy metals induce specicic isozyme patterns of peroxidase inPhaseolus vuigans L. Arch Int Physiol Biochim94:60
Weckx J, Clijsters H (1996) Oxidative damage and defense mee danisms in primary leaves ofPhaselous vulgaris as a result of root assimilation of toxic amounts of copper Physiol Plant96: 506–512
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, UH., Park, JO. Changes in hydrogen peroxide content and activities of antioxidant enzymes in tomato seedlings exposed to mercury. J. Plant Biol. 42, 41–48 (1999). https://doi.org/10.1007/BF03031145
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03031145