Abstract
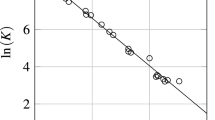
The effect of temperature and pressure on the Gibbs standard free energy formation of ionic species, hence the equilibrium constant involving these ions has been reviewed and discussed. The role of the partial molal volume on the equilibrium constant was reviewed first. It was found that the effect of pressure is negligible unless the pressure applied is excessive, say over 200 atmospheres. The Gibbs standard free energy formation of various metal ions in water at various temperatures has also been calculated using a conventional method as well as the correspondence principles. The temperatures considered were 60, 100. 150 and 200 C. The results obtained for these temperatures based on these methods have been compared and discussed. It has been found that the results by these two methods do not differ much, mostly within 596, which is contrary lo what has been believed by many.
Similar content being viewed by others
References
R. Zena and R. Yeager,J. Phys. Chem. 71, 521 (1967).
D. R. Kestu and R. M. Pytkowicz,Gcoclzim Et. Cosmo. Acfu. 34, 1039 (1970).
G. Curthoys and J. G. Mathieson,Trans. Fur. Soc. 66, 43 (1970).
R. Derry,Miner. Sci. Eng. 4, 3 (1972).
O. J. Kwok and R. G. Robins, inInternatzonal Symposlum on Hydrometallurgy (eds., D. J. I. Evans and R. S. Showmaker), p. 1033, AlME (1972).
C. M. Criss and J. W. Cobble,.J. Am. Chem. Soc. 86. 5385 (1964).
C. M.Criss and J. W. Cobble,.J. Am. Chem. Soc. 86, 6394 (1964).
R. T. lnwson, Potential -pH diagrams above 298.1 f) Part I, Theoretical Background, AAECIE 21 9 (1971).
D. D. Macdonald,Thermodynamic of Metal-water Systems at Elevated Temperatures, Parts 1, 2, 3 and 4, AE-CI- report series (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Han, K.N. Effect of temperature and pressure on equilibrium constant involving metal ions. Metals and Materials 4, 1097–1100 (1998). https://doi.org/10.1007/BF03025982
Issue Date:
DOI: https://doi.org/10.1007/BF03025982