Résumé
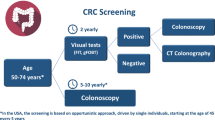
Le cancer colorectal, avec 36000 nouveaux cas et 16000 décès par an en France, est un problème majeur de santé publique. Il justifie le développement de stratégies de dépistage de masse de la population à risque moyen. En France ce dépistage est basé sur la recherche de saignement occulte dans les selles par le test Hémoccult II® répété tous les 2 ans dans 22 des 95 départements Français. D’autres pays développent des stratégies de dépistage endoscopiques. Il est important de comparer ces différentes stratégies en terme de réduction de mortalité, d’incidence du cancer colorectal et en terme de coût-efficacité.
Les études basées sur la réalisation d’un test hémoccult tous les 2 ans mettent en évidence une réduction de mortalité de 14 % après 10 ans de dépistage, moindre après 13 ans et nulle après 16 ans. L’incidence du cancer colorectal n’est pas significativement modifiée après 11 à 16 ans de dépistage.
Les études de dépistage basées sur l’endoscopie mettent en évidence dans les études cas-témoins une réduction de l’incidence du CCR de 45 à 80 % et une réduction de la mortalité de 56 à 75 % surtout au profit des CCR distaux. Les études avec contrôles «historiques» montrent une réduction d’incidence du CCR de 66 à 80 %. Plusieurs résultats d’études contrôlées de dépistage par rectosigmoidoscopie souple sont en attente en 2007–2008.
Les études combinant recherche de saignement occulte et endoscopie ne permettent pas d’augmenter le taux de détection du CCR et ne mettent en évidence qu’une réduction non significative du taux de mortalité.
Le dépistage par sigmoïdoscopie ne permet de détecter que 66% des hommes et 35% des femmes porteurs de CCR. Le risque relatif d’adénome proximal chez les patients porteurs d’adénome distal est de 2,8. Des scores visant à définir la population devant avoir une coloscopie totale après réalisation d’une sigmoïdoscopie ont été élaborés.
Les taux de complications des stratégies de dépistage endoscopiques sont bas, liéà une éventuelle polypectomie comme pour les endoscopies réalisées quelle que soit l’indication. L’acceptabilité du dépistage est meilleure pour le test hémoccult que pour la sigmoïdoscopie et que pour la coloscopie totale et ce quelle que soit les antécédents personnels ou familiaux de cancer colorectal ou d’adénome. La faisabilité du dépistage endoscopique se heurte au problème de la démographie médicale.
D’autres méthodes de dépistage sont décrites: recherche immunologique de sang dans les selles, détection d’ADN fécal, coloscopie virtuelle. Ces études ont prouvées la faisabilité de ces techniques en pratique, mais aucune n’a pour l’instant démontré un intérêt en terme de réduction d’incidence du cancer colorectal et de réduction de mortalité.
Summary
With 36 000 new cases per year and 16 000 deaths per year, colorectal cancer is a major health problem in France. This justifies the development of mass screening strategies for the average risk population. In France mass screening for colorectal cancer is based on fecal occult blood test with Hémoccult II® done every 2-year. This screening program is now applied in 22 of the 95 French administrative areas. Endoscopic screening programs are developed in other countries. It is of importance to compare those different screening program in term of decrease of the colorectal cancer incidence, mortality and in term of cost effectiveness.
Studies based on biennial hemoccult test showed a 16% decrease of colorectal mortality after 10 years of mass screening program. This mortality decrease was lower after 13 years of screening and very limited after 16 years of screening. The incidence of colorectal cancer was not significantly modified after 11 to 16 years of colorectal cancer screening with hemoccult.
Case-control endoscopic-based studies showed a 45 to 80% decrease of colorectal cancer incidence and a 56 to 75% decrease of colorectal cancer mortality, especially for distal colorectal cancers. Trials with historical controlled cohort showed a 66 to 80% decrease of colorectal cancer incidence, without decrease of mortality. Four randomized and controlled studies are now ongoing and results will be available between 2007 and 2008).
Studies with combination of fecal occult blood test and endoscopy didn’t increase the colorectal cancer detection rate and showed no significative decrease of colorectal mortality.
Screening programs based on rectosigmoidoscopy detected only 66% of men and 35% of women with colorectal cancer. The risk of proximal adenoma in the population of persons with distal adenoma detected on rectosigmoidoscopy is 2.8. An index is described to define the population who need a total colonoscopy after rectosigmoidoscopy.
Adverse events rates related on endoscopic screening programs are very low, similar to large endoscopic reports. Acceptability of screening programs is better for hemoccult than for rectosigmoidoscopy and total colonoscopy, even if there is personal or familial history of colorectal cancer or adenoma. Feasibility of endoscopic-based screening programs depends closely to the medical demography.
Others mass screening methods are described: immunological fecal occult blood test, fecal DNA detection, computed-tomography colonography. Those methods are feasible in practice but none have yet showed a decrease of colorectal incidence or mortality.
Similar content being viewed by others
Références
Agence Nationale pour le Développement de l’Evaluation Médicale. Endoscopies digestives basses. Recommandations et références médicales. Gastroenterol Clin Biol 1996; 20: 881–96.
Conférence de Consensus. Prévention, dépistage et prise en charge des cancers du côlon. Gastroenterol Clin Biol 1998; 22: 205–8.
Anonymous. Recommendations on cancer screening in the European Union. Bull Cancer 2001; 88: 687–92.
American Society for Gastrointestinal Endoscopy. Guidelines for CRC screening and surveillance. Gastrointest Endosc 2000; 51: 777–82
American College of Gastroenterology. Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. CRC prevention 2000: screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. Am J Gastroenterol 2000; 95: 868–77.
Anonymous. CRC prevention. Med J Aust 2002; 176: 145–46.
Winawer S, Fletcher R, Rex D, et al for the US Multisociety Task Force on CRC. CRC screening and surveillance: clinical guidelines and rationales-update based on new evidence. Gastroenterology 2003; 124: 544–60.
Selby JV, Friedman GD, Quesenberry CP, Weiss NS. Effect of fecal occult blood testing on mortality from CRC. A case-control study. Ann Intern Med 1993; 118: 1–6.
Faivre J, Tazi MA, El Mrini T, Lejeune C, Benhamiche AM, Dassonville F. Faecal occult blood screening and reduction of CRC mortality: a case-control study. Br J Cancer 1999; 79: 680–3.
Zappa M, Castiglione G, Grazzini G, et al. Effect of fecal occult blood testing on colorectal mortality: result of a population-based case-control study in district of Florence, Italy. Int J Cancer 1997; 73: 208–10.
Warhendorf J, Robra BP, Wiebelt H, Oberhausen R, Weiland M, Dhom G. Effectiveness of CRC screening: results from a population-based case-control study in Saarland, Germany. Eur J Cancer Prev 1993; 2: 221–7.
Lazovich D, Weiss NS, Stevens NG, White E, McKnight B, Wagner EH. A case-control study to evaluate efficacy of screening for fecal occult blood. J Med Screening 1995; 2: 84–9.
Saito H, Soma Y, Koeda J, Wada T, Kawaguchi H, Sobue T. Reduction in risk of mortality from CRC by fecal occult blood screening with immunochemical hemagglutination test. A case-control study. Int J Cancer 1995; 61: 465–9.
Faivre J, Dancourt V, Lejeune C, et al. Reduction in CRC mortality by fecal occult blood screening in a French controlled study. Gastroenterology 2004; 126: 1674–80.
Towler B, Irwig L, Glaszioiu P, Kewenter J, Weller D, Silagy C. A systematic review of the effects of screening for CRC using the faecal occult blood test, Hemoccult. Br Med J 1998; 317: 559–65.
Mandel JS, Bond JH, Church TR, et al for the Minnesota Colon Cancer Control Study. Reducing mortality from CRC by screening for fecal occult blood. N Engl J Med 1993; 328: 1365–71.
Hardcastle JD, Chamberlain JO, Robinson MHE, et al. Randomised controlled trial of fecal-blood screening for CRC. Lancet 1996; 348: 1472–7.
Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for CRC with faecal-occult-blood test. Lancet 1996; 348: 1467–71.
Kewenter J, Brevinge H, Engaras B, Haglind E, Ahren C. Results of screening, rescreening, and follow-up in a prospective randomized study for detection of CRC by fecal occult blood testing. Results for 68,308 subjects. Scand J Gastroenterol. 1994; 29: 468–73.
Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of CRC. N Engl J Med 2000; 343: 1603–7.
Mandel JS, Church TR, Bond JH. CRC mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999; 91: 434–7.
Scholefield JH, Moss S, Sufi F, Mangham CM, Hardcastle JD. Effect of fecal occult blood screening on mortality from CRC: results from a randomized controlled trial. Gut 2002; 50: 840–4.
Jorgensen OD, Kronborg O, Fenger C. A randomised study of screening for CRC using fecal occult blood testing: results after 13 years and seve biennal screening rounds. Gut 2002; 50: 29–32.
Kronborg O, Jorgensen OD, Fenger C, M Ramussen. Randomised study of biennial screening with fecal occult blood testing: results after nine screening rounds. Scand J Gastroenterol 2004; 39: 846–51.
Johnson DA, Gurney MS, Volpe RJ, Jones DM, VanNess MM, Chobanian SJ, Avalos JC, Buck JL, Kooyman G, Cattau EL. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am J Gastroenterol 1990; 85: 969–74.
Rex DK, Lehman GA, Hawes RH, Ulbright TM, Smith JJ. Screening colonoscopy in asymptomatic average-risk persons with negative fecal occult blood tests. Gastroenterology 1991; 100: 64–7.
DiSario JA, Foutch PG, Mai HD, Pardy K, Manne RK. Prevalence and malignant potential of colorectal polyps in asymptomatic, average-risk men. Am J Gastroenterol 1991; 86: 941–5.
Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol 1991; 86: 946–51.
Foutch PG, DiSario JA, Pardy K, Mai HD, Manne RK. The sentinel hyperplastic polyp: a marker for synchronous neoplasia in the proximal colon. Am J Gastroenterol 1991; 86: 1482–5.
Foutch PG, Mai H, Pardy K, DiSario JA, Manne RK, Kerr D. Flexible sigmoidoscopy may be ineffective for secondary prevention of CRC in asymptomatic, average-risk men. Dig Dis Sci 1991; 36: 924–8.
Rex DK, Smith JJ, Ulbright TM, Lehman GA. Distal colonic hyperplastic polyps do not predict proximal adenomas in asymptomatic average-risk subjects. Gastroenterology 1992; 102: 317–9.
Rex DK, Lehman GA, Ulbright TM, Smith JJ, Pound DC, Hawes RH, Helper DJ, Wiersema MJ, Langefeld CD, Li W. Colonie neoplasia in asymptomatic persons with negative fecal occult blood tests: influence of age, gender, and family history. Am J Gastroenterol 1993; 88: 825–31.
Kadakia SC, Wrobleski CS, Kadakia AS, Meier NJ. Prevalence of proximal colonie polyps in average-risk asymptomatic patients with negative fecal occult blood tests and flexible sigmoidoscopy. Gastrointest Endosc 1996; 44: 112–7.
Read TE, Read JD, Butterly LF. Importance of colonie adenomas 5 mm or less in diameter that are detected by sigmoidoscopy. N Engl J Med 1997; 336: 8–12.
Wallace MB, Kemp JA, Trnka YM, Donovan JM, Farraye FA. Is colonoscopy indicated for small adenomas found by screening flexible sigmoidoscopy? Ann Intern Med 1998; 129: 273–8.
Nicholson FB, Korman MG, Stern AI, Hansky J. Distribution of colorectal adenomas: implications for bowel cancer screening. Med J Aust 2000; 172: 428–30.
Ikeda Y, Mori M, Miyazaki M, Yoshizumi T, Maehara Y, Sugimachi K. Significance of small distal adenoma for detection of proximal neoplasms in the colorectum. Gastrointest Endosc 2000; 52: 358–61.
Rosen DM, Sanchez NC, Whitlow CB, Beck DE, Hicks TC, Timmcke AE. Significant findings on screening colonoscopy. Dis Colon Rectum 2003; 46: A51.
Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G for Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for CRC. N Engl J Med 2000; 343: 162–8.
Collet JA, Olynyk JK, Platell CF. Flexible sigmoidoscopy screening for CRC in average-risk people: update of a community-based project. Med J Aust 2000; 173: 463–6.
Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med 2000; 343: 169–74.
Lieberman DA, Weiss DG, for Veterans Affairs Cooperative Study Group 380. One-time screening for CRC with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 2001; 345: 555–60.
Schoenfeld R, Cash B, Flood A, Dobhan R, Eastone J, Coyle W et al. Colonoscopic screening of average risk women for colorectal neoplasia. N Engl J Med 2001; 352: 2061–68.
Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med 2002; 346: 1781–5.
Sung JJY, Chan FKL, Leung WK, et al. Screening for CRC in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology 2003; 124: 608–14.
Harewood GC, Lieberman DA. Prevalence of advanced neoplasia at screening colonoscopy in men in private practice versus academic and veterans affairs medical centers. Am J Gastroenterol 2003; 98: 2312–6.
Wu K, Titzer D, Soetikno R, Triadafilopoulos G. Use of a colonoscope instead of a sigmoidoscope to screen asymptomatic adults for CRC. Gastrointest Endosc 2003; 58: 720–4.
Betes M, Munoz-Navas MA, Duque JM, et al. Use of colonoscopy as a primary screening test for CRC in average risk people. Am J Gastroenterol 2003; 98: 2648–54.
Selby J, Friedman G, Quesenberry CP Jr, Weiss NS. A casecontrol study of screening sigmoidoscopy and mortality from CRC. N Engl J Med 1992; 326: 653–7.
Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and CRC mortality. J Natl Cancer Inst 1992; 84: 1572–5.
Müller A, Sonnenberg A. Prevention of CRC by flexible endoscopy and polypectomy. A case-controlled study of 32,702 veterans. An Intern Med 1995; 123: 904–10.
Müller AD, Sonnenberg A. Protection by endoscopy against death from CRC. A case-control study among veterans. Arch Intern Med 1995; 155: 1741–8.
Kavanagh AM, Giovannucci EL, Fuchs CS, Colditz GA. Screening endoscopy and risk of CRC in United States men. Cancer Causes Control 1998; 9: 455–62.
Slattery ML, Edwards SL, Ma KN, Friedman GD. Colon cancer screening, lifestyle, and risk of colon cancer. Cancer Causes Control 2000; 11: 555–63.
Brenner H, Arndt V, Stürmer T, Stegmaier C, Ziegler H, Dhom G. Long-lasting reduction of risk of CRC following screening endoscopy. Br J Cancer 2001; 85: 972–6.
Newcomb PA, Storer BE, Morimoto LM, Templeton A, Potter JD. Long-term efficacy of sigmoidoscopy in the reduction of CRC incidence. J Natl Cancer Inst 2003; 95: 622–5.
Atkin WS, Morson BC, Cuzick J. Long-term risk of CRC after excision of rectosigmoid adenomas. N Engl J Med 1992; 326: 658–62.
Winawer S J, Stewart ET, Zauber AG, Bond JH, Ansel H, Waye JD, et al. Prevention of CRC by colonoscopic polypectomy. N Engl J Med 1993; 329: 1977–83.
Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M, The Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing CRC incidence. Gut 2001; 48: 812–5.
Brevinge H, Lindholm E, Buntzen S, Kewenter J. Screening for colorectal neoplasia with faecal occult blood testing compared with flexible sigmoidoscopy directly in a 55–56 year old population. Int J Colored Dis 1997; 12: 291–5.
Thiis-Evensen E, Hoff GS, Sauar J, Langmark F, Majak BM, Vatn MH. Population-based surveillance by colonoscopy: effect of the incidence of CRC. Telemark polyp study I. Scand J Gastroenterol 1999; 34: 414–20.
Gohagan JK, Prorok PC, Hayes RB, Kramer BS, for the PLCO Project Team. The prostate, lung, colorectal and ovarian (PLCO) cancer screening trial of the National Cancer Institute: history, organization, and status. Control Clin Trials 2000; 21: 251S-272S.
Bresalier RS, Pinsky P, Weissfeld J, et al. Effectiveness of nurse endoscopists in the prostate, lung, colorectal, and ovarian (PLCO) cancer screening trial (abstract). Gastroenterology 2002; 122: A479.
Segnan N, Senore C, Andreoni B, et al and the Score Working Group. Baseline findings of the Italian multicenter randomized controlled trial of“once-only sigmoidoscopy”-Score (abstract). J Natl Cancer Inst 2002; 94: 1763–72.
Segnan V, Andreoni B, Bisanti L, et al. Comparing different screening strategies for CRC (abstract). Gastroenterology 2002; 122: A483.
Segnan V, Andreoni B, Bisanti L, et al P. Colonoscopy screening for CRC: a comparison study in Italy (abstract). Gastroenterology 2004; 126: A 198.
Segnan N, Senore C, Andreoni B, Arrigoni A, Bisanti L, Cardelli A et al. Randomized trial of different screening strategies for colorectal cancer: patient response and detection rates. J Natl Cancer Inst 2005; 97: 347–57.
Winawer SJ, Zauber AG, Church T, et al. National colonoscopy study preliminary results: a randomized controlled trial of general population screening colonoscopy (abstract). Gastroenterology 2002; 122: A480.
Gondal G, Grotmol T, Hofstad B, Bretthauer M, Eide TJ, Hoff G. The Norwegian CRC prevention (NORCCAP) screening study: baseline findings and implementations for clinical work-up in age groups 50–64 years. Scand J Gastroenterol 2003; 38: 635–42.
UK Flexible Sigmoidoscopy Screening Trial Investigators. Single flexible sigmoidosocpy screening to prevent CRC: baseline findings of a UK multicentre randomised trial. Lancet 2002; 359: 1291–300.
Hoff G, Thiis-Evensen E, Grotmol T, Sauar J, Vatn MH, Moen IE. Do undesirable effect of screening affect all-cause mortality in flexible sigmoidoscopy programmes? Experience from the Telemark polyp study 1983–1996. Eur J Cancer Prevent 2001; 10: 131–7.
Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for CRC with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst 1993; 85: 1311–8.
Rasmussen M, Kronborg O, Fenger C, Jorgensen OD. Possible advantages and drawbacks of adding flexible sigmoidoscopy to Hemoccult-II in screening for CRC. A randomized study. Scand J Gastroenterol 1999; 34: 73–8.
Berry DP, Clarke P, Hardcastle JD, Vellacott KD. Randomized trial of the addition of flexible sigmoidoscopy to faecal occult blood testing for colorectal neoplasia population screening. Br J Surg 1997; 84: 1274–6.
Verne JECW, Aubrey R, Love SB, Talbot IC, Northover JMA. Population based randomised study of uptake and yield screening by flexible sigmoidoscopy compared with screening by faecal occult blood testing. Br Med J 1998; 317: 182–5.
Hoff H, Gondal G, Grotmol T, Hofstad B, Brethauer M, Eide TJ. Adding FOBT to once-only flexible sigmoidoscopy (FS) screening program reduces compliance without improving the yield for significant lesions (abstract). Gastroenterology 2003; 124: A-20.
Rasmussen M, Fenger C, Kronborg O. Diagnostic yield in a biennial Hemoccult-II screening program compared to a onceonly screening with flexible sigmoidoscopy and Hemoccult-II. Scand J Gastroenterol 2003; 38: 114–8.
Achkar E, Carey W. Small polyps found during fiberoptic sigmoidoscopy in asymptomatic patients. Ann Intern Med 1988; 109: 880–3.
Foutch PG, DiSario JA, Pardy K, Mai HD, Manne RK. The sentinel hyperplastic polyp: a marker for synchronous neoplasia in the proximal colon. Am J Gastroenterol 1991; 86: 1482–5.
Rex DK, Lehman GA, Hawes RH, et al. Screening colonoscopy in asymptomatic average-risk persons with negative fecal occult blood tests. Gastroenterology 1991; 100: 64–7.
Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol 1991; 86: 946–51.
Rex DK, Smith JJ, Ulbright TM, Lehman GA. Distal colonic hyperplastic polyps do not predict proximal adenomas in asymptomatic average-risk subjects. Gastroenterology 1992; 102: 317–9.
Brady PG, Straker RJ, McClave SA, Nord HJ, Pinkas M, Robinson BE. Are hyperplastic rectosigmoid polyps associated with an increased risk of proximal colonic neoplasms? Gastrointest Endosc 1993; 39: 481–5.
Zarchy TM, Ershoff D. Do characteristics of adenomas on flexible sigmoidoscopy predict advanced lesions on baseline colonoscopy? Gastroenterology 1994; 106: 1501–4.
Kadakia SC, Wrobleski CS, Kadakia AS, Meier NJ. Prevalence of proximal colonic polyps in average-risk asymptomatic patients with negative fecal occult blood tests and flexible sigmoidoscopy. Gastrointest Endosc 1996; 44: 112–7.
Read TE, Read JD, Butterly LF. Importance of colonic adenomas 5 mm or less in diameter that are detected by sigmoidoscopy. N Engl J Med 1997; 336: 8–12.
Wallace MB, Kemp JA, Trnka YM, Donovan JM, Farraye FA. Is colonoscopy indicated for small adenomas found by screening flexible sigmoidoscopy? Ann Intern Med 1998; 129: 273–8.
Sciallero S, Costantini M, Bertinelli E, et al. Distal hyperplastic polyps do not predict proximal adenomas: results from a multicentric study of colorectal adenomas. Gastrointest Endosc 1997; 46: 124–30.
Schoen RE, Corle D, Cranston L, et al. Is colonoscopy needed for the nonadvanced adenoma found on sigmoidoscopy ? The Polyp Prevention Trial. Gastroenterology 1998; 115: 533–41.
Levin TR, Palitz A, Grossman S, et al. Predicting advanced proximal colonic neoplasia with screening sigmoidoscopy. JAMA 1999; 281: 1611–7.
Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G for Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for CRC. N Engl J Med 2000; 343: 162–8.
Hammer K, Hammer J, Oesterreicher C, Pötzi R. Advanced distal colonie lesions as predictors of advanced lesions in the proximal colon. Medicine 2000; 79: 127–34.
Ikeda Y, Mori M, Miyazaki M, Yoshizumi T, Maehara Y, Sugimachi K. Significance of small distal adenoma for detection of proximal neoplasms in the colorectum. Gastrointest Endosc 2000; 52: 358–61.
Gondal G, Grotmol T, Hofstad B, Bretthauer M, Eide TJ, Hoff G. Grading of distal colorectal adenomas as predictors for proximal colonie neoplasia and choice of endoscope in population screening: experience from the Norwegian CRC Prevention Study. Gut 2003; 52: 398–403.
Pinsky PF, Schoen RE, Weissfeld JL, Bresalier RS, Hayes RB, Gohagan JK. Predictors of advanced proximal neoplasia in persons with abnormal screening flexible sigmoidoscopy. Clin Gastroenterol Hepatol 2003; 1: 103–10.
Betes M, Munoz-Navas MA, et al. Diagnostic value of distal colonie polyps for prediction of advanced proximal neoplasia in an average-risk population undergoing screening colonoscopy. Gastrointest Endosc 2004; 59: 634–41.
Lewis JD, Ng K, Hung KE, et al. Detection of proximal adenomatous polyps with screening sigmoidoscopy: a systematic review and meta-analysis of screening colonoscopy. Arch Intern Med 2003; 163: 413–20.
Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Using risk for advanced proximal colonie neoplasia to tailor endoscopie screening for CRC. Ann Intern Med 2003; 139: 959–65.
Anderson JC, Alpern Z, Messina CR, et al. Predictors of proximal neoplasia in patients without adenomatous pathology. Am J Gastroeneterol 2004; 111: 472–7
Schoenfeld P, Shad J, Ormsteth E, et al. Predictive value of diminutive colonie adenomatrial: the PREDICT••••Clin Gastroenterol Hepatol 2003; 1: 195–201.
Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997; 112: 24–8.
Rex DK, Lehman GA, Ulbright TM, Smith JJ, Hawes RH. The yield of a second screening flexible sigmoidoscopy in average-risk persons after one negative examination. Gastroenterology 1994; 106: 593–5.
Hixson LS, Fennerty MB, Sampliner RE, McGee D, Garewal H. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst 1990; 82: 1769–72.
Atkin WS, Hart A, Edwards R, McIntyre P, Aubrey R, Wardle J, Sutton S, Cuzick J, Northover JMA. Uptake, yield of neoplasia, and adverse effects of flexible sigmoidoscopy screening. Gut 1998; 42: 560–5.
Martinez ME, McPherson RS, Levin B, Glober GA. A case-control study of dietary intake and other lifestyle risk factors for hyperplastic polyps. Gastroenterology 1997; 113: 423–9.
van Stolk RU, Beck GJ, Baron JA, Haile R, Summers R. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. The Polyp Prevention Study Group. Gastroenterology 1998; 115: 13–8.
Noshirwani KC, van Stolk RU, Rybicki LA, Beck GJ. Adenoma size and number are predictive of adenoma recurrence: implications for surveillance colonoscopy. Gastrointest Endosc 2000; 51: 433–7.
Gandhi SK, Reynolds MW, Boyer JG, Goldstein JL. Recurrence and malignancy rates in a benign colorectal neoplasm patient cohort: results of a 5-year analysis in a managed care environment. Am J Gastroenterol 2001; 96: 2761–7.
Lieberman DA, Weiss D. 5-year surveillance of patients with adenomas or CRC at screening colonoscopy: results from the VA cooperative study. Digestive Disease Week and the 105th Annual Meeting of the American Gastroenterological Association — New Orleans, May 15–20, 2004 In: Gastroenterology 2004; 126: A22.
Riff ER, Dehaan K, Garewal GS. The role of sigmoidoscopy for asymptomatic patients. Results of three annual screening sigmoidoscopies, polypectomy, and subsequent surveillance colonoscopy in a primary-care setting. Clev Clin J Med 1990; 57: 131–6.
Krevsky B, Fisher RS. Yield of rescreening for colonie polyps using flexible sigmoidoscopy. Am J Gastroenterol 1994; 89: 1165–8.
Squillace S, Berggreen P, Jaffe P, et al. A normal initial colonoscopy after age 50 does not predict a polyp-free status for life. Am J Gastroenterol 1994; 89: 1156–9.
Neugut AI, Jacobson JS, Ahsan H, et al. Incidence and recurrence rates of colorectal adenomas: a prospective study. Gastroenterology 1995; 108: 402–8.
Rex DK, Cummings OW, Helper DJ, et al. 5-year incidence of adenomas after negative colonoscopy in asymptomatic average-risk persons. Gastroenterology 1996; 111: 1178–81.
Schoen RE, Pinsky PF, Weissfeld JL, et al JK for the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Group. Results of repeat sigmoidoscopy 3 years after a negative examination. Jama 2003; 290: 41–8.
Doria-Rose VP, Levin TR, Selby JV, Newcomb PA, Richert-Boe KE, Weiss NS. The incidence of CRC following a negative screening sigmoidoscopy: implications for screening interval. Gastroenterology 2004; 127: 714–22.
Yamaji Y, Mitsushima T, Ikuma H et al. Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese. Gut 2004; 53: 568–72.
Platell CFE, Phillpott G, Olynyk JK. Flexible sigmoidoscopy screening for colorectal neoplasia in average-risk people: evaluation of a five year rescreening interval. Med J Aust 2002; 176: 371–3.
Waye JD, Kahn O, Auerbach ME. Complications of colonoscopy and flexible sigmoidoscopy. Gastrointest Endosc Clin N Am 1996; 6: 343–77.
Anderson ML, Pasha TM, Leighton JA. Endoscopie perforation of the colon: lessons from a 10-year study. Am J Gastroenterol.2000; 95: 3418–22.
Nelson DB, McQuaid KR, Bond JH, Lieberman DA, Weiss DG, Johnston TK. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc 2002; 55: 307–14.
Levin TR, Conell C, Shapiro JA, Chazan SG, Nadel MR, Selby JV. Complications of screening flexible sigmoidoscopy. Gastroenterology 2002; 123: 1786–92.
Ringel Y, Dalton CB, Brandt LJ, et al. Flexible sigmoidoscopy: the patients’ perception. Gastrointest Endosc 2002; 55: 315–20.
Salkeld G, Solomon M, Short L, Ryan M, Ward JE. Evidencebased consumer choice: a case study in CRC screening. Aust N Z J Public Health 2003; 27: 449–55.
Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003; 349: 2191–200.
Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA 2004; 291: 1713–1719.
Scott RG, Edwards JT, Fritschi L et al. Community-based screening by colonoscopy or computed tomographic colonography in asymptomatic average-risk patient. Am J Gastroenterol 2004;: 1145–51.
Bonithon-Kopp C, Milan C, Pariente EA, Faivre J, Bonaïti-Pellie C. Facteurs influençant l’acceptabilité de la coloscopie chez les apparentés du premier degré de patients atteints d’un gros adénome. Gastroenterol Clin Biol 2002; 26: A48.
Schoen RE, Weissfeld JL, Trauth JM, Ling BS, Hayran M. A population-based, community estimate of total colon examination: the impact on compliance with screening for CRC. Am J Gastroenterol 2002; 97: 446–51.
Pignone M, Bucholtz D, Harris R. Patient preferences for colon cancer screening. J Gen Intern Med 1999; 14: 432–7.
Brenes GA, Paskett ED. Predictors of stage of adoption for CRC screening. Preventive Med 2000; 31: 410–6.
Farraye FA, Wong M, Hurwitz S, et al. Barriers to endoscopic CRC screening: are women different from men? Am J Gastroenterol 2004;342-9.
Turner BJ, Weiner M, Yang C, Tenhave T. Predicting adherence to colonoscopy or flexible sigmoidoscopy on the basis of physician appointment-keeping behaviour. Ann intern Med 2004; 140: 528–532.
Brown ML, Klabunde CN, Mysliwiec P. Current capacity for endoscopic CRC screening in the United States: data from the National Cancer Institute survey of CRC screening practices. Am J Med 2003; 115: 129–33.
Rex DK, Liberman DA. Feasability of colonoscopy screening: Discussion of issues and recommendations regarding implementation. Gastrointes Endosc 2001; 54: 662–7.
Young GP, St John JB, Winawer SJ, Rozen P. Choice of fecal occult blood tests for CRC screening: recommendations based on performance characteristics in population studies. Am J Gastroenterol 2002; 97: 2499–2507.
Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996; 334: 155–9.
Rozen P, Knaani J, Samuel Z. Comparative screening with a sensitive guaiac and specific immunochemical occult blood test in an endoscopie study. Cancer 2000; 89: 46–52.
Zappa M, Castiglione G, Pact E, Grazzini G, Rubeca T, Turco P, Crocetti E, Clatto S. Measuring interval cancers in population-based screening using different assays of fecal occult blood testing: the district of Florence experience. Int J Cancer 2001; 92: 151–4.
Castiglione G, Zappa M, Grazzini G, et al. Immunochemical vs. guaiac fecal occult blood tests in a population based screening program for CRC. Br J Cancer 1996; 65: 942–4.
Launoy GD, Bertrand HJ, Berchi C, Talbourdet VY, Guizard AV, Bouvier VM, Caces ER. Evaluation of an immunochemical fecal occult blood test with automated reading in screening for CRC in a general average-risk population. Int J Cancer 2005; 115:493–6.
Wong WM, Lam SK, Cheung KL, Tong TSM, Rozen P, Young GP, Chu KW, Ho J, Law WL, Tung HM, Choi HK, Lee YM, Lai KC, Hu WHC, Chan CK, Yuen MF, Wong BCY. Evaluation of an automated immunochemical fecal occult blood test for colorectal neoplasia detection in a Chinese population. Cancer 2003; 97: 2420–4.
Ahlquist DA, Skoletsky JE, Boynton KA, Harrington JJ, Mahoney DW, Pierceall WE, Thibodeau SN, Shuber AP. CRC screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology 2000; 119: 1219–27.
Dong SM, Traverso G, Johnson C, Geng L, Favis R, Boynton K, Hibi K, Goodman SN, D’Allessio M, Paty P, Hamilton SR, Sidransky D, Barany F, Levin B, Shuber A, Kinzler KW, Vogelstein B, Jen J. Detecting CRC in stool with the use of multiple genetic targets. J Natl Cancer Inst 2001; 93: 858–65.
Brand RE, Shuber AP, Laken SJ, Young CM, Urbanowski J. Reliability of a stool DNA mutation specific assay for CRC. Digestive Disease Week and the 103rd Annual Meeting of the American Gastroenterological Association — San Francisco, May 19–22,2002. In Gastroenterology 2002; 122: A479.
Traverso G, Shuber A, Levin B, Johnson C, Olsson L, Schoetz DJ Jr, Hamilton SR, Boynton K, Kinzler KW, Vogelstein B. Detection of APC mutations in fecal DNA from patients with colorectal tumors. N Engl J Med 2002; 346: 311–20.
Calistri D, Rengucci C, Bocchini R, Saragoni L, Zoli W, Amadori D. Fecal multiple molecular tests to detect CRC in stool. Clin Gastroenterol Hepatol 2003; 1: 377–83.
Syngal S, Chung D, Willet C. The loss of stool DNA mutation abnormalities in colorectal neoplasia after treatment. Gastroenterology 2003; 24: A5.
Johnson CD, Harmsen WS, Wilson LA, et al. Prospective blinded evaluation of computed tomographic colonography for screen detection of colorectal polyps. Gastroenterology 2003; 125: 311–9.
Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME, for the CRC Study Group. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. New Engl J Med 2004; 351: 2704–14.
Schoenfeld P, Lipscomb S, Crook J, et al. Accuracy of polyp detection by gastroenterologists and nurse endoscopists during flexible sigmoidoscopy: a randomized trial. Gastroenterology 1999; 117: 312–8.
Araujo SE, Habr-Gama A, Nahas SC, Atui FC, Marques CF, Esteves FP. Colonoscopic miss rates of flat and polypoid colorectal lesions determined by magnifying colonoscopy [abstract]. Dis Colon Rectum 2003; 46: A52.
ANAES (agence nationale d’accréditation et de l’évaluation scientifique). Place de la coloscopie virtuelle dans le dépistage du cancer colorectale. Janvier 2001. www, anaes. fr
Sosna J, Morrin MM, Kruskal JB, Lavin PT, Rosen MP, Raptopoulos V. CT colonography of colorectal polyps: a metanalysis. AJR 2003; 181: 1593–8.
Johnson CD, Toledano AY, Herman BA, et al for the American College of Radiology Imaging Network A6656. Computerized tomographic colonography: performance evaluation in a retrospective multicenter setting. Gastroenterology 2003; 125: 688–95.
Pineau BC, Paskett ED, Chen J et al. Virtual colonoscopy using oral contrast compared with colonoscopy for detection of patients with colorectal polyps. Gastroenteroogy 2003; 125: 304–10.
Macari M, Bini EJ, Jacobs SL et al. Colorectal polyps and cancers in asymptomatic average risk patients: evaluation with CT colonography. Radiology 2004; 230: 629–636
Vogt C, Cohen M, Beck et al. Detection of colorectal polyps by multislice CT colongraphy with ultra-low-dose technique: comparison with high-resolution videocolonoscopy. Gastrointest endosc 2004; 60: 201–9.
Rex DK, Vining D, Kopecky KK. An initial experience with screening for colon polyps using spiral CT with and without CT colography (virtual colonoscopy). Gastrointest Endosc 1999; 50: 309–13.
Iannaccone R, Laghi A, Catalano C et al. Detection of colorectal lesions: lower-dose multi-detector row helical CT colonography compared with conventional colonoscopy. Gastroenterology 2004; 229: 775–811.
Edwards JT, Mendelson RM, Fritschi L et al. Colorectal neoplasia screening with CT colonography in average-risk asymptomatic subjects: community-based study. Radiology 2004; 230: 459–64.
Rockey Dc, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, et al. Analysis of air contrast barium enema, computed tomograhic colonography and colonoscopy: prospective comparison. Lancet 2005; 365: 305–11.
Author information
Authors and Affiliations
About this article
Cite this article
Heresbach, D., Manfredi, S. & Bretagne, J.F. Stratégies de dépistage du cancer colorectal: endoscopie versus autres modes d’exploration. Acta Endosc 35, 621–648 (2005). https://doi.org/10.1007/BF03003921
Issue Date:
DOI: https://doi.org/10.1007/BF03003921
Mots-clés
- adénome
- cancer colorectal (CCR)
- coloscopie
- dépistage du cancer colorectal
- hémoccult
- sigmoïdoscopie flexible