Abstract
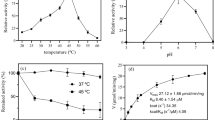
The ability to adherein vitro to urinary catheters and the presence of enterococcal virulence factors was determined in 30Enterococcus urinary isolates (12E. faecalis, 12E. faecium, 3E. casseliflavus, 3E. gallinarum). Silicone, siliconized latex and polyvinyl chloride (PVC) were examined by sonication quantitative culture technique and scanning electron microscope. As compared toE. faecalis andE. faecium, E. casseliflavus andE. gallinarum displayed lower adhesion to all synthetic materials. All the tests performed showed higher adherence of all tested strains to siliconized latex and silicone than to PVC. Biofilmforming ability was observed in 5E. faecalis but in none of the remaining strains. The gene coding enterococcal surface protein (Esp) was detected in 7E. faecalis and 6E. faecium strains. Gelatinase was found in 1E. faecalis, 2E. faecium and hemolysins were found in 6E. faecalis and 1E. faecium strains. AllE. casseliflavus andE. gallinarum strainswere negative for these traits. Hydrophobic type of cell surface (measured by its affinity forn-hexadecane) was shown in a few isolates. Bacterial adherence was not significantly associated with the above pathogenic factors.
Similar content being viewed by others
References
Archimbaud C., Shankar N., Forestier C., Baghdayan A., Gilmore M.S., Charbonne F., Joly B.:In vitro adhesive properties and virulence factors ofEnterococcus faecalis strains.Res.Microbiol. 153, 75–80 (2002).
Baldassarri L., Bertuccini L., Ammendolia M.G., Gherardi G., Creti R.: Variantesp gene in vancomycin-sensitiveEnterococcus faecium.Lancet 357, 1802 (2001a).
Baldassarri L., Cecchini R., Bertuccini L., Ammendolia M.G., Iosi F., Arciola C.R., Montanaro L., Di Rosa R., Gherardi G., Dicuonzo G., Orefici G., Creti R.:Enterococcus spp. produces slime and survives in rat peritoneal macrophages.Med. Microbiol.Immunol. 190, 113–120 (2001b).
Beneš J., Picha D., Kabelková M., Džupová O., Horova B., Gabrielova A.: Infective endocarditis caused by unusual Grampositive pathogens. Report of 4 patients.Folia Microbiol. 47, 737–741 (2002).
Carvalho M.G.S., Teixeira L.M., Facklam R.R.: Use of tests for acidification of methyl β-glucopyranoside and susceptibility to efrotomycin for differentiation of strains ofEnterococcus and some related genera.J.Clin.Microbiol. 36, 1584–1587 (1998).
Cooper G.S., Shlaes D.M., Jacobs M.R.: The role ofEnterococcus in intra-abdominal infections: case control analysis.Infect.Dis. Clin.Pract. 2, 332–339 (1993).
Coque T.M., Patterson J.E., Steckelberg J.M., Murray B.E.: Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons.J.Infect.Dis. 171, 1223–1229 (1995).
Donlan R.M.: Biofilms: microbial life on surfaces.Emerg.Infect.Dis. 8, 881–890 (2002).
Donlan R.M., Costerton J.W.: Biofilms: survival mechanisms of clinically relevant microorganisms.Clin.Mircobiol.Rev. 15, 167–193 (2002).
Eaton T.J., Gasson M.J.: A variant enterococcal surface protein Espfm inEnterococcus faecium; distribution among food, commensal, medical, and environmental isolates.FEMS Microbiol.Lett. 216, 269–275 (2002).
Elsner H.-A., Sobotka I., Mack D., Claussen M., Laufs R., Wirth R.: Virulence factors ofEnterococcus faecalis andEnterococcus faecium blood culture isolates.Eur.J.Clin.Microbiol.Infect.Dis. 19, 39–42 (2000).
Facklam R.R., Sahm D.S., Teixeira L.M.:Enterococcus. pp. 669–682 in L. Collier, A. Balows, M. Sussman (Eds):Topley & Wilson’s Microbiology and Microbial Infections, 9th ed. Edward Arnold, London 1997.
Hammerum A.M., Jensen L.B.: Prevelence ofesp, encoding the enterococcal surface protein, inEnterococcus faecalis andEnterococcus faecium isolates from hospital patients, poultry, and pigs in Denmark.J.Clin.Microbiol. 40, 4396 (2002).
Hirth H., Erlandsen S.L., Dunny G.M.: Heterologous inducible expression ofEnterococcus faecalis pCF10 aggregation substance asc10 inLactococcus lactis to fibrin.J.Bacteriol. 182, 2299–2306 (2000).
Jett B.D., Huycke M.M., Gilmore M.S.: Virulence of enterococci.Clin.Microb.Rev. 7, 462–478 (1994).
Joyanes P., Pascual A., Martinez-Martinez L., Hevia A., Perea E.J.:In vitro adherence ofEnterococcus faecalis andEnterococcus faecium to plastic biomaterials.Clin.Microbiol.Infect. 5, 382–386 (1999).
Joyanes P., Pascual A., Martinez-Martinez L., Hevia A., Perea E.J.:In vitro adherence ofEnterococcus faecalis andEnterococcus faecium to urinary catheters.Eur.J.Clin.Microbiol.Infect.Dis. 19, 124–127 (2000).
Kawalec M., Zaręba T.: Pathogenesis of enterococcal infections.New Medicine 4, 26–30 (1997).
Kreft B., Marre R., Schramm U., Wirth R.: Aggregation substance ofEnterococcus faecalis mediates adhesion to cultured renal tubular cells.Infect.Immun. 60, 25–30 (1992).
Kurup A., Tee W.S.N., Loo L.H., Lin R.: Infection of central nervous system by motileEnterococcus: first case report.J.Clin.Microbiol. 39, 820–822 (2001).
Megran D.W.: Enterococcal endocarditis.Clin.Infect.Dis. 15, 63–71 (1992).
Moellering R.C.J.: Emergence ofEnterococcus as a significant pathogen.Clin.Infect.Dis. 14, 1173–1176 (1992).
Mundy L.M., Sahm D.F., Gilmore M.: Relationships between enterococcal virulence and antimicrobial resistance.Clin.Microbiol. Rev. 13, 513–522 (2000).
Murga R., Miller J.M., Donlan R.M.: Biofilm formation by gram-negative bacteria on central venous catheter connectors: effect of conditioning films in a laboratory model.J.Clin.Microbiol. 39, 2294–2297 (2001).
Neely A.N., Maley M.P.: Survival of enterococci and staphylococci on hospital fabrics and plastic.J.Clin.Microbiol. 38, 724–726 (2000).
Rademaker J.L.W., Louws F.J., De Bruijn F.J.: Characterization of the diversity of ecologically important microbes by rep-PCR genomic fingerprinting, pp. 1–26 in A.D.L. Akkermans, J.D. Van Elsas, F.J. De Brujin (Eds):Molecular Microbial Ecology Manual. Kluwer Academic Publishers, Dordrecht 1997.
Rosenberg M., Gutnick D., Rosenberg E.: Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobocity.FEMS Microbiol.Lett. 9, 29–33 (1980).
Schlievert P.M., Gahr P.J., Assimacopoulos A.P., Dinges M.M., Stoehr J.A., Harmala J.W., Hirth H., Dunny G.M.: Aggregation and binding substances enhance pathogenicity in rabbit models ofEnterococcus faecalis endocarditis.Infect.Immun. 66, 218–223 (1998).
Shankar V., Baghdayan A.S., Huycke M.M., Lindahl G., Gilmore M.S.: Infection-derivedEnterococcus faecalis strains are enriched inesp. a gene encoding a novel surface protein.Infect.Immun. 67, 193–200 (1999).
Shankar N., Lockatell C.V., Baghdayan A.S., Drachenberg C., Gilmore M.S., Johnson D.E.: Role ofEnterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection.Infect.Immun. 69, 4366–4372 (2001).
Süssmuth S.D., Muscholl-Silberhorn A., Wirth R., Susa M., Marre R., Rozdzinski E.: Aggregation substance promotes adherence, phagocytosis, and intracellular survival ofEnterococcus faecalis within human macrophages and suppresses respiratory burst.Infect.Immun. 68, 4900–4906 (2000).
Toledo-Arana A., Valle J., Solano C., Arrizubieta M.J., Cucarella C., Lamata M., Amorena B., Leiva J., Penades J.R., Lasa I.: The enterococcal surface protein, Esp, is involved inEnterococcus faecalis biofilm formation.Appl.Envir.Microbiol. 67, 4538–4545 (2001).
Vergis E.N., Shankar N., Chow J.W., Hayden M.K., Snydman D.R., Zervos M.J., Linden P.K., Wagener M.M., Muder R.R.: Association between the presence of enterococcal virulence factors gelatinase, hemolysin, and enterococcal surface protein and mortality among patients with bacteremia due toEnterococcus faecalis.Clin.Infect.Dis. 35, 570–575 (2002).
Waar K., Muscholl-Silberhorn A.B., Willems R.J.L., Slooff M.J.H., Harmsen H.J.M., Degener J.E.: Genogrouping and incidence of virulence factors ofEnterococcus faecalis in liver transplant patients differ from blood culture and fecal isolates.J.Infect.Dis. 185, 1121–1127 (2002a).
Waar K., Van Der Mei H.C., Harmsen H.J.M., Degener J.E., Busscher H.J.:Enterococcus faecalis surface proteins determine its adhesion mechanism to bile drain materials.Microbiology 148, 1863–1870 (2002b).
Zaręba T., Hryniewicz W.: Clinical significance ofEnterococcus infection.New Medicine 4, 30–33 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dworniczek, E., Kuzko, K., Mróz, E. et al. Virulence factors andin Vitro adherence ofEnterococcus strains to urinary catheters. Folia Microbiol 48, 671–678 (2003). https://doi.org/10.1007/BF02993477
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02993477