Abstract
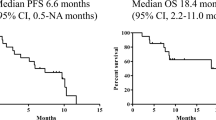
The aim of this study was to evaluate tolerability and efficacy of Leucomax (Sandoz/Schering Plough) used for neutropenia in patients with small cell lung cancer (SCLC) treated with etoposide and cisplatin. The potential influence of granulocyte-macrophage colony stimulating factor (GM-CSF) on chemotherapy relative dose intensity (RDI) was also evaluated. The chemotherapy used was the following, cisplatin 50 mg m-2 i.v. 1 and 7 day, etoposide 170 mg m-2 i.v. 3-5 days, q 3-4 weeks. Patients received a median of six cycles (range 2-8) over 4-36 weeks (median: 20). Thirty-two consecutive patients were treated, six were excluded. Eleven patients received GM-CSF 5 /zg kg"1 s.c. due to absolute neutrophil count (ANC), 1000/mm3 until recovery (ANC > 2000 mm3) or during 7 days, and thereafter prophylactically 24 hours post subsequent chemotherapy cycles for 7 days. Four patients received single GM-CSF course during the terminal disease phase. In 11 patients, there was no neutropenia requiring GM-CSF during the whole treatment course. Toxicity of chemotherapy was high, including thrombocytopenia, neutropenia, anaemia, mucositis, fever and hypotension. GM-CSF toxicity was the following, first dose reaction - one patient, local erythema - two patients, arthralgia - one patient, hypotension, chills, fever requiring GM-CSF discontinuation one patient RDI of cisplatin/etoposide was 0.77/0.62 in GM-CSF group, and 0.90/ 0.80 in patients who didn’t receive Leucomax. Overall objective response rate to chemotherapy and complete response rate were 80% (21/26), 26% (7/26) and median survival of all patients was 10 months. Median disease free survival was 8 months. Four patients are alive, two patients lost during progression, 20 died. Administration of GM-CSF did not appear to improve RDI of chemotherapy, overall response rate (RR) nor survival in this phase I/II clinical study. RDI of chemotherapy was reduced in patients receiving GM-CSF due to thrombocytopenia and/or extrahaematologic toxicity of chemotherapy.
Similar content being viewed by others
References
Johnson, B.E. (1993) Management of small cell lung cancer.Clin. Chest. Med. 14, 173.
Aisner, J.et al. (1986) Doxorubicin, cyclophosphamide, etoposide and platinum for small cell carcinoma of the lung.Sem. Oncol 13 suppl. 3, 54–62.
Evans, W.K., Shepherd, F.A., Feld, R.et al. (1985) VP-16 and cisplatin as a first line therapy for small cell lung cancer.J. Clin. Oncol. 3, 1471–7.
Boni, C, Cocconi, G., Brisgani, G.et al. (1989) Cisplatin and etoposide [VP-16] as a single regimen for small cell lung cancer. A phase II trial.Cancer 63, 638–42.
Ettinger, D.S. (1993) Small cell lung cancer. In J.E. NiederhuberCurrent Therapy in Oncology, p. 182. Mosby-Year Book Inc.
Goldman, A.J. and Goldie, J.H. (1987) Impact of dose intense chemotherapy on the development of permanent drug resistance.Sem. Oncol. 14 (Suppl. 4), 29–33.
Hryniuk, W.M. (1988) The importance of dose intensity in outcome of chemotherapy In V.T. De Vita, S. Hellman and S.A. Rosenberg (eds)Importance Advances in Oncology, pp. 121–142. Philadelphia: JB Lippincott.
Murray, N. (1987) The importance of dose and dose intensity in lung cancer chemotherapy.Sem. Oncol. 14 (Suppl. 4), 20–8.
Durand, R.E. and Goldie, J.H. (1987) Interaction of etoposide and cisplatin in anvitro tumor model.Cancer Treat. Rep. 71, 673–9.
Eder, J.P.O., Teicher, B.A., Holden, S.A.et al. (1990) Ability of four potential topoisomerase II inhibitors to enhance the cytotoxicity of cisdiamminedichloroplatinun [II] in Chinese hamster ovary cells and in an epipodophylotoxin resistance subline.Cancer Chem. Pharmac. 26, 423–8.
Jett, J.R., Everson, L., Therneau, T.M.et al. (1990) Treatment of limited stage small lung cancer with cyclophosphamide, doxorubicin, and vincristine with or without etoposide. A randomized trial of the North Central Cancer Treatment Group.J. Clin. Oncol 8, 33–8.
Wilke, H., Achterrach, W., Schmidt, H.et al. (1988) Etoposide and split dose cisplatin in small cell lung cancer A. M.J. Clin. Oncol 11 (5), 572.
Crawford, J., Ozer, H., Stoller, R.et al. (1991) Reduction by colony-stimulating factor of fever and neutropenia induced by chemotherapy in patient with small cell, lung cancer.N. Eng. J. Med. 325, 164.
WHO Recommendation for grading acute and subacute toxicity. Protocol 20, 921 Version of 30.03.93.
Nemunaitis, J., Singer, J.W., Buckner, C.D.et al. (1988) Use of recombinant human granulocyte-macrophage colony stimulating factor in autologous bone marrow transplantation for lymphoid malignancies.Blood 72, 834–6.
De Vries, E.G., Biesma, B., Willemse, P.et al. (1991) A double blind placebo control study with granulocytemacrophage colony stimulating factor during chemotherapy for ovarian carcinoma.Cancer Res. 51, 116–122.
Idhe, D.C., Mulshine, J.L., Krammer, B.S.et al. (1991) Randomized trial of high versus standard dose etoposide and cisplatin in extensive stage small cell lung cancerProc. ASCO 10, 240 Abstr. 841.
Bodey, G.P., Buckley, M., Sathe, Y.S.et al. (1966) Quantitative relationships between circulating leucocytes and infection in patients with acute leucemia.Ann. Intern. Med. 64, 328–40.
Herrmann, F., Schultz, G., Lindeman, A.et al. (1989) Hematopoietic response in patients with advanced malignancy treated with recombinant human granulocytemacrophage colony stimulating factor.J. Clin. Oncol 7, 159–67.
Gianni, A.M., Bregni, M., Siena, S.et al. (1990) Recombinant human granulocyte-macrophage colony stimulating factor reduces hematologic toxicity and widens clinical applicability of high dose cyclophosphamide treatment in breast cancer and non Hodgkin’s lymphoma.J. Clin. Oncol 8, 768–78.
Phillips, N., Jacobs, S., Stoller, R.et al. (1989) Effect of recombinant granulocyte-macrophage colony stimulating factor on myelopoiesis in patients with refractory metastatic carcinoma.Blood 74, 26–34.
Krigel, R.L., Pelackd, C.S.et al. (1994) Ifosphamide, carboplatin and etoposide plus granulocyte-macrophage colony stimulating factor. A phase I study with apparent activity in non small cell lung cancer.J. Clin. Oncol 6, 1251–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walewski, J., Romejko-Jarosinska, J., Zwolinski, J. et al. Tolerability and efficacy of GM-CSF [Leucomax] in patients with small cell lung cancer treated with intensive chemotherapy. Med Oncol 13, 199–205 (1996). https://doi.org/10.1007/BF02990932
Issue Date:
DOI: https://doi.org/10.1007/BF02990932