Abstract
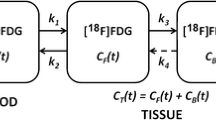
Hyperacute changes in the expression of glycolysis-associate gene products as well as FDG uptake in tumor cells after high-dose irradiation reflect response of the cells to noxious intervention and may be a potential indicator of the outcome of treatment. To understand acute effects on the kinetics of glucose metabolism of tumorsin vivo after high-dose irradiation, we analyzed dynamic FDG PET data in patients with metastatic brain tumors receiving stereotactic radiosurgery.Materials and Methods: We studied 5 patients with metastatic brain tumors by means of dynamic FDG PET before and 4 hours after stereotactic radiosurgery. Rate constants of glucose metabolism (K1 *−k3 *) were determined in a total of 13 tumors by a non-linear least squares fitting method for dynamic PET and arterial blood sampling data. Rate constants after radiosurgery were compared with those before radiosurgery. Changes in the rate constants induced by the therapy were also correlated with changes in tumor size evaluated by CT and/or MRI 6 months later.Results: Four hours after radiosurgery, the phosphorylation rate indicated by k3 * was significantly higher (0.080±0.058) than that before radiosurgery (0.049±0.023) (p<0.05, paired t test), but there was no significant change in the membrane transport rates indicated by K1 * and k2 *. Although increases in the net influx rate constant K* (=K1 *k3 */(k2 *+k3 *)) were correlated with increases in k3 *, K* after radiosurgery (0.027±0.011) was not significantly different from that before the therapy (0.024±0.012). The reduction in the tumor size was correlated with k3 * after radiosurgery.Conclusion: Acceleration of the phosphorylation process was demonstratedin vivo in metastatic brain tumors as early as 4 hours after stereotactic radiosurgery, as shown experimentallyin vitro in a previous report. The phenomenon may be a sensitive indicator of cell damage.
Similar content being viewed by others
References
Iosilevsky G, Front D, Bettman L, Hardoff R, Ben-Arieh Y. Uptake of gallium-67 citrate and [2-3H]deoxyglucose in the tumor model, following chemotherapy and radiotherapy.J Nucl Med 1985; 26: 278–282.
Kitagawa Y, Sadato N, Azuma H, Ogasawara T, Yoshida M, Ishii Y, et al. FDG PET to evaluate combined intraarterial chemotherapy and radiotherapy of head and neck neoplasms.J Nucl Med 1999; 40: 1132–1137.
Rozental JM, Levine RL, Mehta MP, Kinsella TJ, Levin AB, Algan O, et al. Early changes in tumor metabolism after treatment: The effects of stereotactic radiotherapy.Int J Radiat Oncol Biol Phys 1991; 20: 1053–1060.
Rozental JM, Robins HI, Finlay J, Healey B, Levin AB, Steeves RA, et al. Eight-drugs-in-one day chemotherapy in postirradiated adult patients with malignant gliomas.Med Pediatr Oncol 1989; 17: 471–476.
Hautzel H, Müller-Gärtner HM. Early changes in fluorine-18-FDG uptake during radiotherapy.J Nucl Med 1997; 38: 1384–1386.
Maruyama I, Sadato N, Waki A, Tsuchida T, Yoshida M, Fujibayashi Y, et al. Hyperacute changes in glucose metabolism of brain tumors after stereotactic radiosurgery: a PET study.J Nucl Med 1999; 40: 1085–1090.
Fujibayashi Y, Waki A, Sakahara H, Konishi J, Yonekura Y, Ishii Y, et al. Transient increase in glycolytic metabolism in cultured tumor cells immediately after exposure to ionizing radiation: from gene expression to deoxyglucose uptake.Radiat Res 1997; 147: 729–734.
Hartmann GH, Schlegel W, Sturm V, Kober B, Pastyr O, Lorenz WJ Cerebral radiation surgery using moving field irradiation at a linear accelerator facility.Int J Radiat Oncol Biol Phys 1985; 11: 1185–1192.
Lutz W, Winston KR, Maleki N. A system for stereotactic radiosurgery with a linear accelerator.Int J Radiat Oncol Biol Phys 1988; 14: 373–381.
Takayama M, Nakamura M, Ikezaki H, Ikeda I, Kusuda J, Furuya Y, et al. Stereotactic radiosurgery using a linear accelerator (LINAC): simulation and positioning. (Japanese)No Shinkei Geka 1995; 23: 223–228.
Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-d-glucose using aminopolyether supported nucleophilic substitution.J Nucl Med 1986; 27: 235–238.
DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE. Performance characteristics of a whole-body PET scanner.J Nucl Med 1994; 35: 1398–1406.
Kapouleas I, Alavi A, Alves WM, Gur RE, Weiss DW. Registration of three-dimensional MR and PET images of the human brain without markers.Radiology 1991; 181: 731–739.
Uematsu H, Sadato N, Yonekura Y, Tsuchida T, Nakamura S, Sugimoto K, et al. Coregistration of FDG PET and MRI of the head and neck using normal distribution of FDG.J Nucl Med 1998; 39: 2121–2127.
Guerin C, Laterra J, Hruban RH, Brem H, Drewes LR, Goldstein GW. The glucose transporter and blood-brain barrier of human brain tumors.Ann Neurol 1990; 28: 758–765.
Whitmore GF, Till JE, Gwatkin RBL, Siminovich L, Graham AF. Increase of cellular constitutions in X-irradiated mammalian cells.Biochim Biophys Acta 1958; 30: 583–591.
Ng CE, McGovern KA, Wehrle JP, Glickson JD.31P-NMR spectroscopic study of the effects of γ-irradiation on RIF-1 tumor cells perfusedin vitro.Magn Reson Med 1992; 27: 296–309.
Boothman DA, Majmudar G, Johnson T. Immediate X-ray-inducible responses from mammalian cells.Radiat Res 1994; 138: S44–46.
Blacklock JB, Oldfield EH, Di Chiro G, Tran D, Theodore W, Wright DC, et al. Effect of barbiturate coma on glucose utilization in normal brain versus gliomas. Positron emission tomography studies.J Neurosurg 1987; 67: 71–75.
Ishizu K, Nishizawa S, Yonekura Y, Sadato N, Magata Y, Tamaki N, et al. Effects of hyperglycemia on FDG uptake in human brain and glioma.J Nucl Med 1994; 35: 1104–1109.
Rozental JM, Levine RL, Nickles RJ. Changes in glucose uptake by malignant gliomas: preliminary study of prognostic significance.J Neurooncol 1991; 10: 75–83.
Ishikawa M, Kikuchi H, Nagata I, Yamagata S, Taki W, Yonekura Y, et al. Glucose consumption and rate constants for18F-fluorodeoxyglucose in human gliomas.Neurol Med Chir (Tokyo) 1990; 30: 377–381.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, T., Nishizawa, S., Maruyama, I. et al. Acute effects of stereotactic radiosurgery on the kineties of glucose metabolism in metastatic brain tumors: FDG PET study. Ann Nucl Med 15, 103–109 (2001). https://doi.org/10.1007/BF02988599
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02988599