Abstract
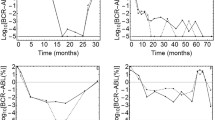
We previously showed that Wilms tumor gene (WT1) expression level, measured by quantitative reverse transcriptase polymerase chain reaction (RT-PCR), was useful as an indicator of minimal residual disease (MRD) in leukemia and myelodysplastic syndrome. However, in conventional quantitative RT-PCR (CQ-PCR), RT-PCR must be performed for various numbers of cycles depending onWT1 expression level. In the present study, we developed a new real-time quantitative RT-PCR (RQ-PCR) method for quantitatingWT1 transcripts. Results of intraassay and interassay variability tests demonstrated that the real-timeWT1 assay had high reproducibility.WT1 expression levels measured by the RQ- and the CQ-PCR methods were strongly correlated (r = 0.998). Furthermore, a strong correlation was observed amongWT1 transcript values normalized with 3 different control genes (β-actin,ABL, andglyceraldehyde-3-phosphate dehydrogenase) and between relativeWT1 transcript values withWT1 expression in K562 cells as the reference and absoluteWT1 transcript copy numbers per microgram RNA. WhenWT1 expression andminor bcr-abl expression were concurrently monitored in 2 patients withbcr-abl-positive acute lymphoblastic leukemia, both MRDs changed mostly in parallel, indicating the reliability and validity of our RQ-PCR method. In conclusion, this RQ-PCR method is convenient and reliable for monitoring MRD and enables routine clinical use of aWT1 assay.
Similar content being viewed by others
References
Call KM, Glaser T, Ito CY, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus.Cell. 1990;60:509–520.
Kreidberg JA, Sariola H, Loring JM, et al.WT1 is required for early kidney development.Cell. 1993;74:679–691.
Reddy JC, Licht JD. TheWT1 Wilms’ tumor suppressor gene: how much do we really know?Biochim Biophys Acta. 1996;1287:1–28.
Inoue K, Sugiyama H, Ogawa H, et al.WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia.Blood. 1994;84:3071–3079.
Bergmann L, Miething C, Maurer U, et al. High levels of Wilms’ tumor gene (WT1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome.Blood. 1997;90:1217–1225.
Inoue K, Ogawa H, Yamagami T, et al. Long-term follow-up of minimal residual disease in leukemia patients by monitoringWT1 (Wilms tumor gene) expression levels.Blood. 1996;88:2267–2278.
Tamaki H, Ogawa H, Inoue K, et al. Increased expression of the Wilms tumor gene (WT1) at relapse in acute leukemia [letter].Blood. 1996;88:4396–4398.
Tamaki H, Ogawa H, Ohyashiki K, et al. The Wilms’ tumor geneWT1 is a good marker for diagnosis of disease progression of myelodysplastic syndromes.Leukemia. 1999;13:393–399.
Holland PM, Abramson RD, Watson R, Gelfand DH. Detection of specific polymerase chain reaction product by utilizing the 5′ → 3′ exonuclease activity of Thermus aquaticus DNA polymerase.Proc Natl Acad Sci U S A. 1991;88:7276–7280.
Lee LG, Connell CR, Bloch W. Allelic discrimination by nick-translation PCR with fluorogenic probes.Nucleic Acids Res. 1993;21:3761–3766.
Livak KJ, Flood SJ, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization.PCR Methods Appl. 1995;4:357–362.
Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR.Genome Res. 1996;6:986–994.
Buonamici S, Ottaviani E, Testoni N, et al. Real-time quantitation of minimal residual disease in inv(16)-positive acute myeloid leukemia may indicate risk for clinical relapse and may identify patients in a curable state.Blood. 2002;99:443–449.
Mensink E, van de Locht A, Schattenberg A, et al. Quantitation of minimal residual disease in Philadelphia chromosome positive chronic myeloid leukaemia patients using real-time quantitative RT-PCR.Br J Haematol. 1998;102:768–774.
Sato S, Honma Y, Hozumi M, et al. Effects of herbimycin A and its derivatives on growth and differentiation of Ph1-positive acute lymphoid leukemia cell lines.Leuk Res. 1994;18:221–228.
Lossos IS, Jones CD, Wamke R, et al. Expression of a single gene, BCL-6, strongly predicts survival in patients with diffuse large B-cell lymphoma.Blood. 2001;98:945–951.
Osumi K, Fukui T, Kiyoi H, et al. Rapid screening of leukemia fusion transcript in acute leukemia by real-time PCR.Leuk Lymphoma. 2002;43:2291–2299.
Ogawa H, Tamaki H, Ikegame K, et al. The usefulness of monitoringWT1 gene transcripts for the prediction and management of relapse following allogeneic stem cell transplantation in acute type leukemia.Blood. 2003;101:1698–1704.
Marcucci G, Livak KJ, Bi W, Strout MP, Bloomfield CD, Caligiuri MA. Detection of minimal residual disease in patients with AML1/ETOassociated acute myeloid leukemia using a novel quantitative reverse transcription polymerase chain reaction assay.Leukemia. 1998;12:1482–1489.
Cassinat B, Zassadowski F, Balitrand N, et al. Quantitation of minimal residual disease in acute promyelocytic leukemia patients with T(15;17) translocation using real-time RT-PCR.Leukemia. 2000;14:324–328.
Pongers-Willemse MJ, Verhagen OJ, Tibbe GJ, et al. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific Taq-Man probes.Leukemia. 1998;12:2006–2014.
Donovan JW, Ladetto M, Zou G, et al. Immunoglobulin heavychain consensus probes for real-time PCR quantification of residual disease in acute lymphoblastic leukemia.Blood. 2000;95:2651–2658.
Gaiger A, Schmid D, Heinze G, et al. Detection of theWT1 transcript by RT-PCR in complete remission has no prognostic relevance in de novo acute myeloid leukemia.Leukemia. 1998;12:1886–1894.
Elmaagacli AH, Beelen DW, Trenschel R, Schaefer UW. The detection ofWT1 transcripts is not associated with an increased leukemic relapse rate in patients with acute leukemia after allogeneic bone marrow or peripheral blood stem cell transplantation.Bone Marrow Transplant. 2000;25:91–96.
Sykes PJ, Neoh SH, Brisco MJ, Hughes E, Condon J, Morley AA. Quantitation of targets for PCR by use of limiting dilution.Biotechniques. 1992;13:444–449.
Gilliland G, Perrin S, Blanchard K, Bunn HF. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction.Proc Natl Acad Sci U S A. 1990;87:2725–2729.
Kreuzer KA, Saborowski A, Lupberger J, et al. Fluorescent 5′- exonuclease assay for the absolute quantification of Wilms’ tumour gene (WT1) mRNA: implications for monitoring human leukaemias.Br J Haematol. 2001;114:313–318.
Siehl JM, Thiel E, Leben R, Reinwald M, Knauf W, Menssen HD. Quantitative real-time RT-PCR detects elevated Wilms tumor gene (WT1) expression in autologous blood stem cell preparations (PBSCs) from acute myeloid leukemia (AML) patients indicating contamination with leukemic blasts.Bone Marrow Transplant. 2002;29:379–381.
Cilloni D, Gottardi E, De Micheli D, et al. Quantitative assessment ofWT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients.Leukemia. 2002;16:2115–2121.
Shichishima T, Okamoto M, Ikeda K, et al. HLA class II haplotype and quantitation ofWT1 RNA in Japanese patients with paroxysmal nocturnal hemoglobinuria.Blood. 2002;100:22–28.
Miyoshi Y, Ando A, Egawa C, et al. High expression of Wilms’ tumor suppressor gene predicts poor prognosis in breast cancer patients.Clin Cancer Res. 2002;8:1167–1171.
Radich J, Gehly G, Lee A, et al. Detection ofbcr-abl transcripts in Philadelphia chromosome-positive acute lymphoblastic leukemia after marrow transplantation.Blood. 1997;89:2602–2609.
Suzuki T, Higgins PJ, Crawford DR, et al. Control selection for RNA quantitation.Biotechniques. 2000;29:332–337.
Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays.J Mol Endocrinol. 2000;25:169–193.
Lossos IS, Czerwinski DK, Wechser MA, Levy R. Optimization of quantitative real-time RT-PCR parameters for the study of lymphoid malignancies.Leukemia. 2003;17:789–795.
Lion T. Debate round table on RT-PCR controls: concluding remarks and mini-review.Leukemia. 2001;15:1033–1037.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tamaki, H., Mishima, M., Kawakami, M. et al. Monitoring Minimal Residual Disease in Leukemia Using Real-time Quantitative Polymerase Chain Reaction for Wilms Tumor Gene (WT1). Int J Hematol 78, 349–356 (2003). https://doi.org/10.1007/BF02983561
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02983561