Abstract
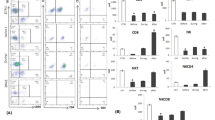
The present study was performed to examine whether the expression of CD64 Fc gamma receptor type I (FC7RI) on both neutrophils and monocytes can be modulated by multiple daily administrations of granulocyte colony-stimulating factor (G-CSF) to patients with non-Hodgkin's lymphoma in neutropenia caused by CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy. The expression of CD64 was determined by flow cytometric analysis at the following time points: before chemotherapy, at the nadir of the neutrophil count, at the fifth day after the start of G-CSF administration, and at more than 8 days after the start of G-CSF administration. CD64 expression was enhanced in patients given G-CSF during CHOP treatment, whereas CD64 expression remained unchanged in patients not given G-CSF. CD64 expression levels on both neutrophils and monocytes were significantly up-regulated by the daily administration of G-CSF and reached peak levels at day 5 (P =.0007). Thereafter, expression on both cell types remained at almost the same levels as on day 5 for the rest of the treatment course, even though G-CSF therapy continued for 3 to 5 more days. Interestingly, CD64 expression on monocytes was already increased significantly (P =.0001) at the nadir of the neutrophil count relative to the baseline before chemotherapy and then was additionally up-regulated by day 5 after the start of G-CSF injections (P =.019). In antibody-dependent cellular cytotoxicity assays, we found that rituximab-mediated cell lysis was significantly enhanced at day 5 after the start of G-CSF treatment (P =.01). In conclusion, this study shows that multiple doses of G-CSF administered to lymphoma patients with neutropenia due to CHOP chemotherapy can enhance CD64 expression on both neutrophils and monocytes. Peak CD64 levels are reached at day 5 of G-CSF treatment, resulting in an activation of the rituximab-mediated antitumor ability of these effector cells. This finding may be useful in determining the optimal timing of administration for an antibody such as rituximab in a chemotherapeutic strategy designed to exert a maximal effect against tumor cells.
Similar content being viewed by others
References
Reff ME, Camer K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20.Blood. 1994;83:435–445.
Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation.Blood. 1984;63:1424–1433.
Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes.Immunol Today. 1994;15:450–454.
Press OW, Appelbaum F, Ledbetter JA, et al. Monoclonal antibody 1F5 (anti-CD20) serotherapy of human B cell lymphomas.Blood. 1987;69:584–591.
Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies.Blood. 1998;91:1644–1652.
Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis.Blood. 2000;95:3900–3908.
Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms.Scand J Immunol. 2000;51:634–641.
Idusogie EE, Presta LG, Gazzano-Santoro H, et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc.J Immunol. 2000;164:4178–4184.
Anderson DR, Grillo-Lopez A, Varns C, Chambers KS, Hanna N. Targeted anti-cancer therapy using rituximab, a chimeric anti- CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin’s B-cell lymphoma.Biochem Soc Trans. 1997;25:705–708.
Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets.Nat Med. 2000;6:443–446.
Voso MT, Pantel G, Rutella S, et al. Rituximab reduces the number of peripheral blood B-cells in vivo mainly by effector cell-mediated mechanisms.Haematologica. 2002;87:918–925.
Deo YM, Graziano RF, Repp R, van de Winkel JG. Clinical significance of IgG Fc receptors and Fc-yR-directed immunotherapies.Immunol Today. 1997;18:127–135.
Hoffmeyer F, Witte K, Schmidt RE. The high-affinity Fc gamma RI on PMN: regulation of expression and signal transduction.Immunology. 1997;92:544–552.
Elsasser D, Valerius T, Repp R, et al. HLA class II as potential target antigen on malignant B cells for therapy with bispecific antibodies in combination with granulocyte colony-stimulating factor.Blood. 1996;87:3803–3812.
Repp R, Valerius T, Sendler A, et al. Neutrophils express the high affinity receptor for IgG (FcγRI, CD64) after in vivo application of recombinant human granulocyte colony-stimulating factor.Blood. 1991;78:885–889.
Valerius T, Repp R, de Wit TPM, et al. Involvement of the high- affinity receptor for IgG (Fc gamma RI; CD64) in enhanced tumor cell cytotoxicity of neutrophils during granulocyte colony-stimulating factor therapy.Blood. 1993;82:931–939.
Kerst JM, de Haas M, van der Schoot CE, et al. Recombinant granulocyte colony-stimulating factor administration to healthy volunteers: induction of immunophenotypically and functionally altered neutrophils via an effect on myeloid progenitor cells.Blood. 1993;82:3265–3272.
Stockmeyer B, Valerius T, Repp R, et al. Preclinical studies with FcγR bispecific antibodies and granulocyte colony-stimulating factor-primed neutrophils as effector cells against HER-2/neu overexpressing breast cancer.Cancer Res. 1997;57:696–701.
Kerst JM, van de Winkel JGJ, Evans AH, et al. Granulocyte colonystimulating factor induces hFc gamma RI (CD64 antigen)-positive neutrophils via an effect on myeloid precursor cells.Blood. 1993;81:1457–1464.
Michon J, Moutel S, Barbet J, et al. In vitro killing of neuroblastoma cells by neutrophils derived from granulocyte colony-stimulating factor-treated cancer patients using an anti-disialoganglioside/anti- FcγRI bispecific antibody.Blood. 1995;86:1124–1130.
Maloney DG, Grillo-Lopez AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma.J Clin Oncol. 1997;15:3266–3274.
Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma.Blood. 1997;90:2188–2195.
Tobinai K, Kobayashi Y, Narabayashi M, et al. Feasibility and pharmacokinetic study of a chimeric anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) in relapsed B cell lymphoma: the IDEC- C2B8 Study Group.Ann Oncol. 1998;9:527–534.
McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program.J Clin Oncol. 1998;16:2825–2833.
Hainsworth JD, Burris HA III, Morissey LH, et al. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma.Blood. 2000;95:3052–3056.
Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation.Blood. 2001;97:101–106.
Czuczman MS, Grillo-Lopez AJ, White CA, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy.J Clin Oncol. 1999;17:268–276.
Vose JM, Link BK, Grossbard ML, et al. Phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated aggressive non-Hodgkin's lymphoma.J Clin Oncol. 2001;19:389–397.
Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma.N Engl J Med. 2002;346:235–242.
Michon JM, Gey A, Moutel S, et al. In vivo induction of functional FcγRI (CD64) on neutrophils and modulation of blood cytokine mRNA levels in cancer patients treated with G-CSF (rMetHuG- CSF).Br J Haematol. 1998;100:550–556.
Schmidt RE, Perussia B. Cluster report: CD16. In: Knapp W, Dörken B, Gilks WR, et al, eds. Leucocyte Typing IV, White Cell Differentiation Antigens. Oxford, UK: Oxford University Press. 1989:574–578.
Stroncek DF, Jaszcz W, Herr GP, Clay ME, McCullough J. Expression of neutrophil antigens after 10 days of granulocyte-colonystimulating factor.Transfusion. 1998;38:663–668.
Ohsaka A, Saionji K, Kuwaki T, Takeshima T, Igari J. Granulocyte colony-stimulating factor administration modulates the surface expression of effector cell molecules on human monocytes.Br J Haematol. 1995;89:465–472.
Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor.Blood. 1991;78:2791–2808.
Nicola NA. Hemopoietic cell growth factors and their receptors.Ann Rev Biochem. 1989;58:45–77.
Lord BI, Molineux G, Pojda Z, Souza LM, Mermod J-J, Dexter TM. Myeloid cell kinetics in mice treated with recombinant interleukin-3, granulocyte colony-stimulating factor (CSF), or granulocyte-macrophage CSF in vivo.Blood. 1991;77:2154–2159.
Gericke GH, Ericson SG, Pan L, Mills LE, Guyre PM, Ely P. Mature polymorphonuclear leukocytes express high-affinity receptors for IgG (FcγRI) after stimulation with granulocyte colony-stimulating factor (G-CSF).J Leukoc Biol. 1995;57:455–461.
Bovolenta C, Gasperini S, Cassatella MA. Granulocyte colony- stimulating factor induces the binding of STAT 1 and STAT 3 to the IFNγ response region within the promoter of the FcγRI/CD64 gene in human neutrophils.FEBS Lett. 1996;386:239–242.
Stockmeyer B, Schiller M, Repp R, et al. Enhanced killing of B lymphoma cells by granulocyte colony-stimulating factor-primed effector cells and Hu1D10—a humanized human leucocyte antigen DR antibody.Br J Haematol. 2002;118:959–967.
van der Kolk LE, de Haas M, Grillo-Lopez AJ, Baars JW, van Oers MHJ. Analysis of CD20-dependent cellular cytotoxicity by G-CSF-stimulated neutrophils.Leukemia. 2002;16:639–699.
Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene.Blood. 2002;99:754–758.
Weber JS, Yang JC, Topalian SL, Schwartzentruber DJ, White DE, Rosenberg SA. The use of interleukin 2 and lymphokine-activated killer cells for the treatment of patients with non-Hodgkin’s lymphoma.J Clin Oncol. 1992;10:33–40.
Friedberg JW, Neuberg D, Gribben JG, et al. Combination immunotherapy with rituximab and interleukin 2 in patients with relapsed or refractory follicular non-Hodgkin's lymphoma.Br J Haematol. 2002;117:828–834.
Davis TA, Maloney DG, Grillo-Lopez CA, et al. Combination immunotherapy of relapsed or refractory low-grade or follicular non-Hodgkin's lymphoma with rituximab and interferon-α-2a.Clin Cancer Res. 2000;6:2644–2652.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kakinoki, Y., Kubota, H. & Yamamoto, Y. CD64 Surface Expression on Neutrophils and Monocytes Is Significantly Up-Regulated after Stimulation with Granulocyte Colony-Stimulating Factor during CHOP Chemotherapy for Patients with Non-Hodgkin’s Lymphoma. Int J Hematol 79, 55–62 (2004). https://doi.org/10.1007/BF02983535
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02983535