Abstract
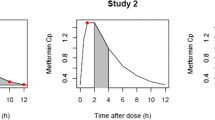
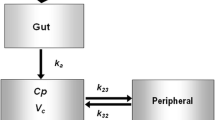
Metformin is a biguanide antihyperglycemic agent often used for the treatment of non-insulin dependent diabetics (NIDDM). In this study, the pharmacokinetics and pharmacodynamics of metformin were investigated in Korean healthy volunteers during a fasting state for over 10 h. In order to evaluate the amount of glucose-lowering effect of metformin, the plasma concentrations of glucose were measured for a period of 10 h followed by the administration of metformin (oral 500 mg) or placebo. In addition, the concentration of metformin in blood samples was determined by HPLC assay for the drug. All volunteers were consumed with 12 g of white sugar 10 minutes after drug intake to maintain initial plasma glucose concentration. The time courses of the plasma concentration of metformin and the glucose-lowering effect were analyzed by nonlinear regression analysis. The estimated Cmax, Tmax, CL/F (apparent clearance), V/F(apparent volume of distribution), and half-life of metformin were 1.42 ± 0.07 μg/mL, 2.59 ± 0.18 h, 66.12 ± 4.6 L/h, 26.63 L, and 1.54 h respectively. Since a significant counterclock-wise hysteresis was found for the metformin concentration in the plasma-effect relationship, indirect response model was used to evaluate pharmacodynamic parameters for metformin. The mean concentration at half-maximum inhibition IC50, kin, and kuot were 2.26 μg/mL, 83.26 h-1, and 0.68 h-1 respectively. Therefore, the pharmacokinetic-pharmacodynamic model may be useful in the description for the relationship between plasma concentration of metformin and its glucose-lowering effect.
Similar content being viewed by others
References
Dayneka, N. L., Garg, V., and Jusko, W. J., Comparison of four basic models of indirect pharmacodynamic responses.J. Pharmacokin. Biopharm., 21, 457–478 (1993).
Kwon, K. I., Clinical Drug Therapy of Disease, the 2nd Ed., Shin II, pp. 433-437 (2003).
McEvoy, G. K. and Livak, K. G., AHFS Drug Information®, ASPH, pp. 3052-3061 (2002).
Shargel, L. and Yu, A. B. C., Relationship between pharmacokinetics and pharmacodynamics, Chapter 19 of Applied Biopharmaceutics and Pharmacokinetics. The 4th Ed., Appleton and Lange, pp. 573-606 (1999).
Stepensky, D. and Fredman, M., Preclinical evaluation of pharmacokinetic-pharmacodynamic rationale for oral CR metformin formulation.J. Con. Rel., 71, 107–115 (2001).
Walker. R. and Edwards, C., Clinical Pharmacyand Therapeutics, the 2nd Ed., Churchill Livingstone, pp. 633-636 (2003).
Walsh, P., Physicians’ Desk Reference, the 55th Ed, Medical Economics, pp. 1079-1085 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.H., Kwon, Ki. Pharmacokinetic-pharmacodynamic modeling for the relationship between glucose-lowering effect and plasma concentration of metformin in volunteers. Arch Pharm Res 27, 806–810 (2004). https://doi.org/10.1007/BF02980152
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02980152