Abstract
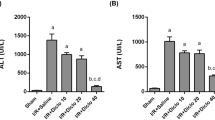
Hepatic ischemia and reperfusion (l/R) predisposes the liver to secondary stresses such as endotoxemia, possiblyvia dysregulation of the hepatic microcirculation secondary to an imbalanced regulation of the vascular stress genes. In this study, the effect of hepatic l/R on the hepatic vasoregulatory gene expression in response to endotoxin was determined. Rats were subjected to 90 min of hepatic ischemia and 6 h of reperfusion. Lipopolysaccharide (LPS, 1 mg/kg) was injected intraperitoneally after reperfusion. Plasma and liver samples were obtained 6 h after reperfusion for serum aminotransferase assays and RT-PCR analysis of the mRNA for the genes of interest: endothelin-1 (ET-1), its receptors ETA and ETB, endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS), heme oxygenase-1 (HO-1), cyclooxygenase-2 (COX-2), and tumor necrosis factor-α (TNF-a). The activities of serum aminotransferases were significantly increased in the l/R group. This increase was markedly potentiated by LPS treatment. The ET-1 mRNA was increased by LPS alone, and this increase was significantly greater in both the l/R alone and l/R + LPS groups compared to the sham. There were no significant differences in ETA receptor mRNA levels among any of the experimental groups. ETB mRNA was increased by both LPS alone and l/R alone, with no significant difference between the l/R alone and l/R + LPS groups. The eNOS and HO-1 transcripts were increased by l/R alone and further increased by l/R + LPS. The iNOS mRNA levels were increased by l/R alone, but increased significantly more by both LPS alone and l/R + LPS compared to l/R alone. The TNF-a mRNA levels showed no change with l/R alone, but were increased by both LPS alone and l/R + LPS. The COX-2 expression was increased significantly by l/R alone and significantly more by l/R + LPS. Taken collectively, significantly greater induction of the vasodilator genes over the constriction forces was observed with l/R + LPS. These results may partly explain the increased susceptibility of ischemic livers to injury as a result of endotoxemia.
Similar content being viewed by others
References
Anderson, B. O. and Harken A. H., Multiple organ failure: Inflammatory priming and activation sequences promote autologous tissue injury.J. Trauma, 30, S44-S49 (1990).
Bauer, M., Bauer I., Sonin, N. V., Kresge, N., Baveja, R., Yokoyama, Y., Harding, D., Zhang, J. X., and Clemens, M. G., Functional significance of endothelin B receptors in mediating sinusoidal and extrasinosoidal effects of endothelins in the intact rat liver.Hepatology, 31, 937–947 (2000).
Bucher, M. and Taeger, K., Endothelin-receptor gene expression in rat endotoxemia.Intensive Care Med., 28, 642–647 (2002).
Bulger, E. M., Garcia, I., and Marier, R. V., Induction of hemeoxygenase 1 inhibits endothelial cell activation by endotoxin and oxidant stress.Surgery, 134, 146–152 (2003).
Chromczynski, R. and Sacchi, N., Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction.Anal. Biochem., 162, 156–159 (1987).
Clemens, M. G., Bauer, M., Gingalewski, C., Miescher, E., and Zhang, J. X., Hepatic intercellular communication in shock and inflammation.Shock, 2, 1–9 (1994).
Colletti, L. M. and Green, M., Lung and liver injury following hepatic ischemia/reperfusion in the rat is increased by exogenous lipopolysaccharide which also increases hepatic TNF productionin vivo andin vitro.Shock, 16, 312–319 (2001).
Dawiskiba, J., Zimecki, M., Kwiatkowska, D., and Kuzminski, A., The effect of endotoxin administration on cytokine production in obstructive jaundiced rats.Arch. Immunol. Ther. Exp. (Warsz), 49, 391–397 (2001).
Dinchuck, J. E., Car, B. D., Frocht, R. J., Johnston, J. F., Jaffee, B. D., Covington, M. B., and Contel, N. R., Renal abnormalities and altered inflammatory response in mice lacking cyclooxygenase-2.Nature, 378, 406–409 (1995).
Fleming, I., Julou-Schaeffer, G., Gray, G. A., Parratt, J. R., and Stoclet, J. C., Evidence that an L-arginine/nitric oxide dependent elevation of tissue cyclic GMP content is involved in depression of vascular reactivity by endotoxin.Br. J. Pharmacol., 103, 1047–1052 (1991).
Heemann, U., Szabo, A., Hamar, P., Muller, V., Witzke, O., Lutz, J., and Philipp, T., Lipopolysaccharide pretreatment protects from renal ischemia/reperfusion injury: Possible connection to an interleukin-6-dependent pathway.Am. J. Pathol., 156, 287–293 (2000).
Housset, C., Rockey, D. C., and Bissell, D. M., Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin-1.Proc. Natl. Acad. Sci. U.S.A., 90, 9266–9270 (1993).
Kahlke, V., Dohm, C., Mees, T., Brotzmann, K., Schreiber, S., and Schroder, J., Early interleukin-10 treatment improves survival and enhances immune function only in males after hemorrhage and subsequent sepsis.Shock, 18, 24–28 (2002).
Kim, Y.-H. and Lee, S.-M., Role of Kupffer cells in vasoregulatory gene expression during hepatic ischemia/ reperfusion.Arch. Pharm. Res., 27, 111–117 (2004).
Lefer, A. M. and Lefer D. J., Nitric oxide II. Nitric oxide protects in intestinal inflammation.Am. J. Physiol., 276, G572-G575 (1999).
Meakins, J. L., Etiology of multiple organ failure.J. Trauma, 30, S165-S168 (1990).
Nakano, J., Takizawa, H., Ohtoshi, T., Shoji, S., Yamaguchi, M., Ishii, A., and Yanagisawa, M., Endothelial and proinflammatory cytokines stimulate endothelin-1 expression and release by airway epithelial cells.Exp. Allergy, 24, 330–336 (1994).
Nevalainen, T. J., Arminger, L. C., and Gavin, J. B., Effects of ischaemia on vasculature.J. Mol. Cell Cardiol., 18, 7–10 (1986).
Ohuchi, T., Tada, K., and Akamatsu, K., Endogenous ET-1 contributes to liver injury induced by galactosamine and endotoxin in isolated perfused rat liver.Am. J. Physiol., 268, G997-G1003 (1995).
Rocky, D. C. and Chung, J. J., Reduced nitric oxide production 72 by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension.Gastroenterology, 114, 344–351 (1998).
Pannen, B. H., Bauer, M., Zhang, J. X., Robotham, J. L., and Clemens, M. G., A time-dependent balance between endothelins and nitric oxide regulating portal resistance after endotoxin.Am. J. Physiol., 271, H1953-H1961 (1996).
Stephenson, K., Gandhi, C. R., and Olson, M. S., Biological actions of endothelin.Vit. Horm., 48, 157–198 (1994).
Thurman, R. G., Marzi, I., Seitz, G., Thies, J., Lemasters, J. J., and Zimmerman, F., Hepatic reperfusion injury following orthotopic liver transplantation in the rat.Transplantation, 46, 502–506 (1988).
Yokoyama, Y., Baveja, R., Kresge, N., Sonin, N., Nakanishi, K., Zhang, J. X., Gitzelmann, C. A., and Clemens M. G., Endothelin receptor remodeling induces the portal venous hyper-response to endothelin-1 following endotoxin pretreatment.Shock, 17, 36–40 (2002).
Zhang, J. X., Pegoli, W., and Clemens, M. G., Endothelin-1 induces direct constriction of hepatic sinusoids.Am. J. Physiol., 266, G624-G632 (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SH., Lee, SM. Expression of hepatic vascular stress genes following ischemia/reperfusion and subsequent endotoxemia. Arch Pharm Res 27, 769–775 (2004). https://doi.org/10.1007/BF02980147
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02980147