Abstract
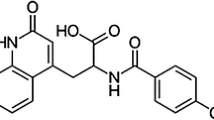
Four crystal forms of ketorolac have been obtained by recrystallization in organic solvents under variable conditions. Different ketorolac polymorphs and pseudopolymorph were characterized by X-ray powder diffraction crystallography (XRD), Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). In the dissolution studies in water at 37+−0.5°C, four crystal forms showed different patterns. The solubility of Form I were the highest. The solubility decreased in rank order: Form I > Form II > Form III > Form IV. Form land Form III were shown to have a good physical stability at room temperature for 60 days. However, Form II is converted to Form III and Form IV is converted to Form I after 60 days storage. Therefore, these observations indicate that crystalline polymorphism for ketorolac is readily inter-convertible and the relationship may have to taken into consideration in the formulation of the drug.
Similar content being viewed by others
References
Borka, L., Reviews on Crystal Polymorphism of Substances in the European Pharmacopoeia.Pharm. Acta Helv., 66, 16–22 (1991).
Haleblian, J. and McCrone, W., Pharmaceutical Applications of polymorphism.J. Pharm. Sci., 58, 911–929 (1969).
Haleblian, J., Characterization of Habits and Crystalline Modification of Solids and their Pharmaceutical Application.J. Pharm. Sci., 64, 1269–1288 (1975).
Kuhnert-Brandstaetter, M., Polymorphic von Arzneistoffen und ihre Bedeutung in der Pharmazeutiscen Technologie.Informationsdienst A.P.V., 19, 73–90 (1973).
Lachman, L. and Lieberman, H. A., Kanig, J. L. (Eds.): The Theory & Practice of Industrial Pharmacy, Lea & Febiger, Philadelphia. 171–196, 1986.
Shefter, E. and Higuchi, T., The Influence of Hydrate and Solvate Formation on Rates of Solution and Solubility of Drugs.J. Pharm. Sci., 52, 781–791 (1963).
Sohn, Y.T., Pharmaceutical Application of Polymorphism.Pharmacon., 21, 500–516 (1991).
Sohn, Y.T. and Kim, K.S., Study on Polymorphism of Cimetidine.J. Kor. Pharm. Sci., 23, 81–87 (1993).
Sohn, Y. T., The Effect of Pseudopolymorphism on the Relative Bioavailability of Amoxicillin.Yakhak Hoeji, 39, 438–443 (1995).
Sohn, Y. T. and Lee, E. H., Crystal Forms of Cefazolin Sodium Hydrates.Yakhak Hoeji, 40, 306–310 (1996).
Sohn, Y. T. and Kim, S. Y., Effect of Crystal Form onin Vivo Topical Anti-Inflammatory Activity of Corticosteroids.Arch. Pharm. Res., 25, 556–559 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sohn, YT., Seo, H.O. Crystal forms of ketorolac. Arch Pharm Res 27, 357–360 (2004). https://doi.org/10.1007/BF02980073
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02980073