Abstract
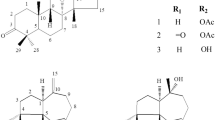
(+)-Lyoniresinol-3α-O-β-d-glucopyranoside (1) was isolated from an ethyl acetate extract of the root bark fromLycium chinense Miller, and its structure was determined using 1D and 2D NMR spectroscopy including DEPT, HMQC, and HMBC. (+)-Lyoniresinol-3α-O-β-d-glucopyranoside exhibited potent antimicrobial activity against antibiotic-resistant bacterial strains, methicillinresistantStaphylococcus aureus (MRSA) isolated from patients, and human pathogenic fungi without having any hemolytic effect on human erythrocytes. In particular, compound1 induced the accumulation of intracellular trehalose onC. albicans as stress response to the drug, and disrupted the dimorphic transition that forms pseudo-hyphae caused by the pathogenesis. This indicates that (+)-lyoniresinol-3α-O-β-d-glucopyranoside has excellent potential as a lead compound for the development of antibiotic agents.
Similar content being viewed by others
References
Achenbach, H., Löwel, M., Waibel, R., Gupta, M., and Solis, P., New lignan glucosides fromStemmadenia minima.Planta Med., 58, 270–272 (1992).
Alvarez-Peral, F. J. and Arguelles, J.-C., Changes in external trehalase activity during human serum-induced dimorphic transition inCandida albicans.Res. Microbiol., 151, 837–843 (2000).
Blondle, S. E. and Houghten, R. A., Design of model amphipathic peptides having potent antimicrobial activities.Biochemistry, 31, 12688–12694 (1992).
Funayama, S., Yoshida, K., Konno, H., and Hikkino, H., Structure of Kukoamine A, a hypotensive principle ofLycium chinense root bark.Tetrahedron Lett., 21, 1355–1356 (1980).
Funayama, S., Zhang, G.-R., Nozoe, S., and Kukoamine, B., A spermine alkaloid fromLycium chinense.Phytochemistry, 38, 1529–1531 (1995).
Han, S.-H., Lee, H.-H., Lee, I.-S., Moon, Y.-H., and Woo, E.-R., A new phenolic amide fromLycium chinense Miller.Arch. Pharm. Res., 25, 433–437 (2002).
Kim, S. Y., Choi, Y.-H., Huh, H., Kim, J., Kim, Y. C., and Lee, H. S., New antihepatotoxic cerebroside fromLycium chinense Fruits.J. Nat. Prod., 60, 274–276 (1997).
Lehrer, R., Lichtenstein, A. K., and Ganz, T., Defensins: Antimicrobial and cytotoxic peptides of mammalian cells.Annu. Rev. Immunol., 11, 105–128 (1993).
Lee, D. G., Park, Y., Kim, M.-R., Jung, H. J., Seu, Y. B., Hahm, K.-S., and Woo, E.-R., Antifungal effects of phenolic amides isolated from the root bark ofLycium chinense.Biotechnol. Lett., 26, 1125–1130 (2004).
Mclain, N., Ascaniom, R., Baker, C., Strohaver, R. A., and Dolan, J. W., Undeclenic acid inhibits morphogenesis ofCandida albicans.Antimicrob. Agents Chemothr., 44, 2873–2875 (2000).
Morota, T., Sasaki, H., Chin, M., Sato, T., Katayama, N., Fukuyama, K., and Mitsuhashi, H., Studies on the crude drug containing the angiotensin I converting enzyme inhibitors (I) on the active principles ofLycium chinense Miller.Shoyakugaku Zasshi, 41, 169–173 (1987).
Sannai, A., Fujimori, T., and Kato, K., Isolation of (−)-1,2-dehydro-α-cyperone and solavetivone fromLycium chinense.Phytochemistry, 21, 2986–2987 (1982).
Sengupta, S., Jana, M. L., Sengupta, D., and Naskar, A. K., A note on the estimation of microbial glycosidase activities by dinitrosalicylic acid reagent.Appl. Microbiol. Biotechnol., 53, 732–735 (2000).
Terauchi, M., Kanamori, H., Nobuso, M., Yahara, S., and Nohara, T., Detection and determination of antioxidative components inLycium chinense.Nat. Med., 51, 387–391
Terauchi, M., Kanamori, H., Nobuso, M., Yahara, S., and Yamasaki, K., New acyclic diterpene glycoside, Lyciumoside IV–IX fromLycium chinense Mill.Nat. Med., 52, 167–171 (1998).
Yahara, S., Shigeyama, C., Ura, T., Wakamatsu, K., Yasuhara, T., and Nohara, T., Cyclic peptides, acyclic diterpene glycoside and other compounds fromLycium chinense Mill.Chem. Pharm. Bull., 41, 703–709 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, D.G., Jung, H.J. & Woo, ER. Antimicrobial property of (+)-lyoniresinol-3α-O-β-d-Glucopyranoside isolated from the root bark ofLycium chinense Miller against human pathogenic microorganisms. Arch Pharm Res 28, 1031–1036 (2005). https://doi.org/10.1007/BF02977397
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977397