Abstract
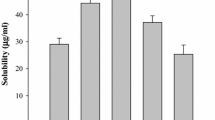
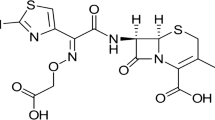
The objective of this study was to elucidate the feasibility to improve the solubility and bioavailability of poorly water-soluble itraconazolevia solid dispersions by using supercritical fluid (SCF). Solid dispersions of itraconazole with hydrophilic polymer, HPMC 2910, were prepared by the aerosol solvent extraction system (ASES) under different process conditions of temperature/pressure. The particle size of solid dispersions ranged from 100 to 500 nm. The equilibrium solubility increased with decrease (15 to 10 MPa) in pressure and increase (40 to 60°C) in temperature. The solid dispersions prepared at 45°C/15 MPa showed a slight increase in equilibrium solubility (approximately 27-fold increase) when compared to pure itraconazole, while those prepared at 60°C/10 MPa showed approximately 610-fold increase and no endothermic peaks corresponding to pure itraconazole were observed, indicating that itraconazole might be molecularly dispersed in HPMC 2910 in the amorphous form. The amorphous state of itraconazole was confirmed by DSC/XRD data. The pharmacokinetic parameters of the ASES-processed solid dispersions, such as Tmax, Cmax, and AUC0–24h were almost similar to Sporanox® capsule which shows high bioavailability. Hence, it was concluded that the ASES process could be a promising technique to reduce particle size and/or prepare amorphous solid dispersion of drugs in order to improve the solubility and bioavailability of poorly water-soluble drugs.
Similar content being viewed by others
References
Bleich, J. and Muller, B. W., Production of drug loaded microparticles by the use of supercritical gases with the aerosol solvent extraction system (ASES) process.J. Microencapsul., 13, 131–139 (1996).
Bustami, R. T., Chan, H., Sweeney, T., Dehghani, F., and Foster, N. R., Generation of fine powders of recombinant human deoxyribonuclease using the aerosol solvent extraction system.Pharm. Res., 20, 2028–2035 (2003).
Colette, B. L. E., Geert, V., and Dany, T., Antifungal compositions with improved bioavailability. Patent WO 97-44014 (1997).
De Beule K. and Van Gestel, J., Pharmacology of itraconazole.Drugs, 61, 27–37 (2001).
Elvassore, N., Baggio, M., Pallado, P., and Bertucco, A., Production of different morphologies of biocompatible polymeric materials by supercritical CO2 antisolvent techniques.Biotechnol. Bioeng., 73, 449–457 (2001).
Engwicht, A., Girreser, U., and Muller, B. W., Critical properties of lactide-co-glycolide polymers for the use in microparticle preparation by the aerosol solvent extraction system.Int. J. Pharm., 185, 61–72 (1999).
Ghaderi, R., Artursson, P., and Carlfors, J., Preparation of biodegradable microparticles using solution-enhanced dispersion by supercritical fluids (SEDS).Pharm. Res., 16, 676–681 (1999).
Harwood, R. J., Hydroxypropylmethylcellulose, Handbook of Pharmaceutical Expients 3rd edition, American Pharmaceutical Association/The Pharmaceutical Press, p. 252–255 (2000).
Jung, J. and Perrut, M., Particle design using supercritical fluids: Literature and patent survey.J. Supercrit. Fluid, 20, 179–219 (2001).
Jung, J. Y., Yoo, S. D., Lee, S. H., Kim, K. H., Yoon, D. S., and Lee, K. H., Enhanced solubility and dissolution rate of itraconazole by a solid dispersion technique.Int. J. Pharm., 187, 209–218 (1999).
Kitamura, M., Yamamoto, M., Yoshinaga, Y., and Masuoka, H., Crystal size control of sulfathiazole using high pressure carbon dioxide.J. Cryst. Growth., 178, 378–386 (1997).
Kohri, N., Yamayoshi, Y., Xin, H., Iseki, K., Sato, N., Todo, S., and Miyazaki, K., Improving the oral bioavailability of albendazole in rabbits by the solid dispersion technique.J. Pharm. Phamacol., 51, 159–164 (1999).
Kordikowski, A., Shkunov, T., and York, P., Polymorph control of sulfathizole in supercritical CO2.Pharm. Res., 18, 682–688 (2001).
Leuner, C. and Dressman, J., Improving drug solubility for oral delivery using solid dispersions.Eur. J. Pharm. Biopharm., 50, 47–60 (2000).
Marr, R. and Gamse, T., Use of supercritical fluids for different processes including new developments-a review.Chem. Eng. Proc., 39, 19–28 (2000).
Martin, T. M., Bandi, N., Shulz, R., Roberts C. B., and Kompella, U. B., Preparation of Budesonide and Budesonide-PLA Microparticles Using Supercritical Fluid Precipitation Technology.AAPS Pharm. Sci. Teh., 3(3): article 18 (2002).
Miyake, K., Irie, T., Arima, H., Hirayama, F., Uekama, K., Hirano, M., and Okamaoto, Y., Characterization of itraconazole/2-hydroxypropyl-beta-cyclodextrin inclusion complex in aqueous propyleneglycol solution.Int. J. Pharm., 179, 237–245 (1999).
Moneghini, M., Kikic, I., Voinovich, D., Perissutti, B., and Filipovic-Grcic, J., Processing of carbamazepine-PEG 4000 solid dispersions with supercritical carbon dioxide: preparation, characterisation, andin vitro dissolution.Int. J. Pharm., 222, 129–138 (2001).
Okimoto, K., Miyake, M., Ibuki, R., Yamasumura, M., Ohnish, N., and Nakai, T., Dissolution mechanism and rate of solid dispersion particles of nilvadipine with hydroxypropyl methylcellulose.Int. J. Pharm., 159, 85–93 (1997).
Palakodaty, S. and York, P., Phase behavioral effects on particle formation processes using supercritical fluids.Pharm. Res. 16, 976–985 (1997).
Palakodaty, S., York, P., and Pritchard, J., Supercritical fluid processing of materials from aqueous solutions: the application of SEDS to lactose as a model substance.Pharm. Res., 15, 1835–1843 (1998).
Paul, M. V., Valentine, F. V., and Roger, P. G., Beads having a core coated with an antifungal and a polymer. Patent WO 94-05263 (1994).
Randolph, T. W., Randolph, A. D., Mebes, M. and Yeung, S., Sub-micrometer-sized biodegradable particles of poly(l-lactic acid)via the gas antisolvent spray precipitation process.Biotechnol. Prog., 9, 429–435 (1993).
Shin-Etsu Catalogue, USP Hydroxypropyl Methylcellulose, “PHARMACOAT” Film Coating Materil and Binder, No 95.9/1,000, Shin-Etsu Chemical Co., Ltd.
Stevens, D. A., Itraconazole in cyclodextrin solution.Pharmacotherapy, 19, 603–611 (1999).
Suzuki, H. and Sunada, H., Influence of water-soluble polymers on the dissolution of nifedipine solid dispersions with combined carriers.Chem. Pharm. Bull., 46, 482–487 (1998).
Tom, J. W. and Debenedetti, P. G., Formation of bioerodible polymeric microspheres and microparticles by rapid expansion of supercritical solutions.Biotechnol. Prog., 7, 403–411 (1991).
Uch, A. S., Hesse, U., and Dressman, J. B., Use of 1-methyl-pyrrolidone as a solubilizing agent for determining the uptake of poorly soluble drugs.Pharm. Res., 16, 968–671 (1999).
Willems, L., van der Geest, R., and de Beule, K., Itraconazole oral solution and intravenous formulations: a review of pharmacokinetics and pharmacodynamics.J. Clin. Pharm. Therap., 26, 159–169 (2001).
Woo, J. S., Antifungal oral composition containing itraconazole and process for preparing same. US Patent 6,039,981 (2000).
Yeo, S. D., Debendetti, P. G., Patro, S. Y., and Przybycien, T. M., Secondary structure characterization of microparticulate insulin powders.J. Pharm. Sci., 83, 1651–1656 (1994).
Yoo, S. D., Lee, S. H., Kang, E., Jun, J., Jung, J. Y., Park, J. W., and Lee, K. H., Bioavailability of itraconazole in rats and rabbits after administration of tablets containing solid dispersion particles.Drug Dev. Ind. Pharm., 26, 27–34 (2000).
Young, T. J., Mawson, S., Johnston, K. P., Henriksen, I. B., Pace, G. W., and Mishra, A. K., Rapid expansion from supercritical to aqueous solution to produce submicron suspensions of water-insoluble drugs.Biotechnol. Prog., 16, 402–407 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S., Nam, K., Kim, M.S. et al. Preparation and characterization of solid dispersions of itraconazole by using aerosol solvent extraction system for improvement in drug solubility and bioavailability. Arch Pharm Res 28, 866–874 (2005). https://doi.org/10.1007/BF02977355
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977355