Abstract
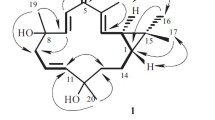
Phytochemical investigation of the methanolic extract of the aerial parts ofLippia nodiflora Linn, led to the isolation of a new triterpenoid lippiacin (1) and a benzofuranone rengyolone (halleridone,2) through repeated silica gel column chromatography and semi preparative HPLC. The structures of these compounds were determined by spectroscopic methods, including 1D and 2D NMR, as well as by comparison with published data. A complete assignment of the1H- and13C-NMR data of2 is reported based on 2DNMR (COSY-45,HMQC and HMBC) spectroscopic methods.
Similar content being viewed by others
References
Basu, A. K., Chakraborti, P., and Sanyal, P. K., Nodifloretin-A new flavone from itLippi nodifloria.J. Ind. Chem. Soc., 46, 271–272 (1969).
Bianco, A., Scalzo, R. L., and Scarpati, M. L., Isolation of cornoside fromOlea europaea and its transformation into halleridone.Phytochemistry, 23, 455–457 (1993).
Budzikiewicz, H., Wilson, J. M., and Djerassi, C., Mass spectrometry in structural and stereo chemical problems pentacyclic triterpenes.J. Am. Chem. Soc., 85, 3688- 3699 (1963).
Chopra, R. N., Nayar, S. L., and Chopra, I. C., Glossary of Indian Medicinal Plants, Council of Scientific and Industrial Research, New Delhi, p.155 (1956).
Endo, K. and Hikino, H., Structures of rengyol, rengyoxide, and rengyolone, new cyclo-hexylethane derivatives fromForsythia suspense fruit.Can. J. Chem., 62,2011 (1984).
Forestieri, A. M., Monforte, M. T., Ragusa, S., Trovato, A., and Lauk, L., Antiinflammatory, analgesic and antipyretic activity in rodents of plant used in African medicine.Phytoth. Res., 10, 100–106 (1996).
Francisco, A., Barbaran, T., Harborne, B. J., and Self, R., Twelve 6-oxygenated flavone sulphates fromLippia nodiflora andL. canescens.Phytochemistry, 26, 2281–2284 (1987).
Hooker, J. D., The Flora British India,L. Reve and Co. 5, Henrietta Street, Covent Garden, London, Vol. IV, P. 563 (1885).
Jayaweera, D. M. A., Medicinal Plants (Indigenous and Exotic Used in Ceylon), The National Science Council of Srilanka, Colombo, Part V, p. 169 (1982).
Kirtikar, K. R., The Indian Medicinal plants, Sudhindra Nath Basu, M.B. Panini: Office 13 Bhuwaneswari Asrama Bahadur Gang, Allahabad, Part-11, pp.986–987 (1918).
Knight, S. A., Carbon-13 NMR spectra of some tetra- and pentacyclic triterpenoids.Org. Mag. Reson., 6, 603–611 (1974).
Manjunath, B. L., The Wealth of India, Council of Scientific and Industrial Research, New Delhi, India, Vol. VI, pp. 142–143 (1962).
Messana, I., Sperandei, M., Multari, G., Galeffi, C., and Bettolo, G. B. M., A cyclohexadie-none and cyclohexenone fromHalleria lucida.Phytochemistry, 23, 2617–2619 (1984).
Nair, A. G. R., Ramesh, P., Nagarajan, S., and Subramanian, S., New flavone glycosides fromLippia nodifloria.Ind. J. Chem., 2, 1316–1317 (1973).
Riaz, N., Anis, I., Rehman, A., Malik, A., Ahmed, Z., Muhammad, P., Shujaat, S., and Rahaman, A., Emodinol, β-Glucuronidase inhibiting triterpene fromPaeonia emodi.Nat. Prod. Res., 17, 247–251 (2003).
Seo, S., Tomita, Y., and Tori, K., Biosynthesis of oleanene- and ursene- type triterpenes from [4-13C] mevalonolactone and [1, 2-13C2] acetate in tissue cultures ofIsodon japonicus HaraJ. Am. Chem. Soc., 103, 2075–2080 (1981).
Siddiqui, B. S., llyas, F., Rasheed, M., and Begum, S., Chemical constituents of leaves and stem bark ofPlumeria obtuse.Phytochemistry, 65, 2077–2084 (2004).
Tori, K., Seo, S., Shimaoka, A., and Tomita, Y., Carbon-13 NMR spectra of olean-12-enes. Full signal assignment including quaternary carbon signals assigned by use of indirect13C,1H spin couplings.Tet. Lett., 48, 4227–4230 (1974).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siddiqui, B.S., Ahmad, F., Sattar, F.A. et al. Chemical constituents from the aerial parts ofLippia nodiflora linn.. Arch Pharm Res 30, 1507–1510 (2007). https://doi.org/10.1007/BF02977318
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977318