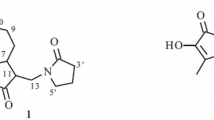
Abstract
Phytochemical works on the aerial parts ofAster oharai (Compositae) led to the isolation of a new sesquiterpene hydroperoxide, 7α-hydroperoxy-3, 11-eudesmadiene (2) and seven known compounds, teucdiol B (1), α-spinasterol (3), oleanolic acid (4), α-spinasterol 3-O-β-D-glucopyranoside (5), methyl 3,5-di-O-caffeoyl quinate (6), 3,5-di-O-caffeoylquinic acid (7), 3,4-di-O-caffeoylquinic acid (8). The chemical structures of1–8 were established by chemical and spectroscopic methods. Compound2 showed cytotoxicity against cultured human tumor cell linesin vitro, SK-OV-3 (ovarian), SK-MEL-2 (skin melanoma), and HCT15 (colon) with ED50 values ranging from 3.86-17.21 ug/mL.
Similar content being viewed by others
References
Ahmad, V. U. and Rahman, A. U., Handbook of natural products data; Pentacyclic triterpenoids.Elsevier Science, 2, 111 (1994).
Basnet, P., Matsushige, K., Hase, K., Kadota, S., and Namba, T., Four di-O-caffeoyl quinic acid derivatives from Propolis. Potent hepatoprotective activity in experimental liver injury models.Biol. Pharm. Bull., 19, 1479–1484 (1996).
Bohlmann, F., Zeisberg, R., and Klien, E., Naturally occurring terpene derivatives. L. Carbon-13 NMR spectra of monoterpenes.Org. Mag. Res., 7, 426–432 (1975).
Choi, S. Z., Kwon, H. C., Choi, S. U., and Lee, K. R., Five New Labdane Diterpenes fromAster oharai.J. Nat. Prod., 65, 1102–1106 (2002).
Fraga, B. M., Hernandez, M. G., Mestres, T., Arteaga, J. M., and Perales, A., Eudesmane sesquiterpenes fromTeucrium heterophyllum. The X-ray structure of teucdiol A.Phytochemistry, 34, 1083–1086 (1993).
Fraga, B. M., Hernandez, M. G., Mestres, T., Terrero, D., and Arteaga, J. M., Nor-sesquiterpenes fromTeucrium heterophyllum.Phytochemistry, 39, 617–619 (1995).
Goad, L. J., Analysis of sterols. Blackie academic & professional, London, pp. 388 (1997).
Iida, T., Ishikawa, T., Tamura, T., and Matumoto, T., Carbon-13 nuclear magnetic resonance spectroscopic evidence of chondrillasterol isolated from ground seed oil.Yukagaku, 29, 345–346 (1980).
Iwahashi, H., Morishita, H., Osaka, N., and Kido, R., 3-O-Feruloyl-4-O-caffeoylquinic acid fromcoffee beans.Phytochemistry, 24, 630–632 (1985).
Kim, T. J., Wild flowers of Korea. Seoul, Korea, Kugilmedia, p. 232, (1996).
Lee, K. R., Peroxide constituents in the natural product research.Kor. J. Pharmacogn., 22, 145–155 (1991).
Lin, L. C., Kuo, Y. C., and Chou, C. J., Immunomodulatory principles ofDichrocephala bicolor.J. Nat. Prod., 62, 405–408 (1999).
Lu, T., Vargas, D., and Fischer, N. H., Sesquiterpenes fromBrintonia discoidea.Phytochemistry, 34, 737–742 (1993).
Mahato, S. B. and Kundu, A. P.,13C-NMR spectra of pentacyclic triterpenoids-a complilation and some salient features.Phytochemistry, 37, 1517–1575 (1994).
Oliveira, F. C., Ferreira, M. J. P., Nunez, C. V., Rodriguez, G. V., and Emerenciano, V. P.,13C-NMR spectroscopy of eudesmane sesquiterpenes.Prog. Nucl. Mag. Res. Sp., 7, 145 (2000).
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J. T., Bokesch, H., Kenney, S., and Boyd, M. R., New colorimetric cytotoxicity assay for anticancer-drug screening.J. Natl. Cancer Inst., 82, 1107–1112 (1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, S.Z., Lee, S.O., Choi, S.U. et al. A new sesquiterpene hydroperoxide from the aerial parts ofAster oharai . Arch Pharm Res 26, 521–525 (2003). https://doi.org/10.1007/BF02976874
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02976874