Abstract
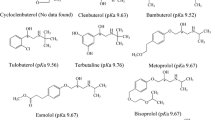
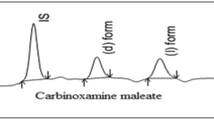
(1′R, 2R)-, (1′R, 2S)-, (1′S, 2R)- and (1′S, 2S)-α-hydroxymetoprolol; (2R)- and (2S)-O-desmethylmetoprolol; and (2R)- and (2S)-metoprolol acid are major metabolites of (2R)-and (2S)-metoprolol, β-adrenergic antagonist. The focus of most chiral separation methods until now has been on determination of the enantiomeric parent drug. However, it is just as important to be able to follow the metabolism of the enantiomers and their possible chiral metabolites. Therefore, for the study of stereoselective metabolism and pharmacokinetic of metoprolol, the chiral separation of the enantiomers of metoprolol and its metabolites has been investigated using four chiral stationary phases, i.e., Chiralcel OD, Chiral-AGP, Cyclobond I and Sumichiral OA-4900 columns. Metoprolol acid was resolved only by Sumichiral OA-4900. Chiralcel OD provided the highest separation factor and resolution value for metoprolol and O-desmethylmetoprolol and partially resolved the four stereoisomers of α-hydroxymetoprolol. Diastereomeric α-hydroxymetoprolols were resolved using the coupled column chromatographic system of two chiral stationary phases, Sumichiral OA-4900 column and Chiralcel OD column.
Similar content being viewed by others
References
Baldwin, J. J. and Abrams, W. B., In: Drug Stereochemistry. New York, Marcel Dekker, 319 (1988).
Balmer, K., Persson, A., Lagerstrom, P. O. and Persson, B. A., Liquid chromatographic separation of the enantiomers of metoprolol and its α-hydroxy metabolite on Chiralcel OD for determination in plasma and urine.J. Chromatogr., 553, 391–397 (1991).
Borg, K. O., Carlsson, E., Hoffmann, K. J., Jonsson, T. E., Thorin, H. and Wallin, B., Metabolism of metoprolol [3H] in man, the dog and the rat.Acta Pharmacol. Toxicol., 36, 125–135 (1975).
Ekelund, J., Arkens, A., Kirsten, B. H., Fich, K., Olsen, L and Peterson, P. V., Chiral separations of β-blocking drug substances using chiral stationary phases.J. Chromatogr. A, 708, 253–261 (1995).
Hermansson, J. and Grahn, A., Optimization of the separation of enantiomers of basic drugs. Retention mechanisms and dynamic modification of the chiral bonding properties on an α1-acid glycoprotein column.J. Chromatogr. A, 694, 57–69 (1995).
Kim, K. H., Choi, B. W., Hong, S. and Kim, H. J., Chiral purity test of metoprolol enantiomer after derivatization with (−)-menthyl chloroformate by reversed-phase high performance liquid chromatography.Arch. Pharm. Res., 22, 614–618 (1999).
Kim, M. S., Shen, D. D., Eddy, A. C. and Nelson, W. L., Inhibition of the enantiselective oxidative metabolism of metoprolol by verapamil in human liver microsomes.Drug Metab. Dispos., 21, 309–317 (1992).
Leloux, M. S., Rapid chiral separation of metoprolol in plasma-Application to the pharmacokinetics/pharmaco-dynamics of metoprolol enantiomers in the conscious goat. Biomed.Chromatogr., 6, 99–105 (1992).
Li, F., Cooper, S. F. and Cote, M., Determination of the enantiomers of metoprolol and its major acidic metabolite in human urine by high-performance liquid chromatography with fluorescence detection.J. Chromatogr. B, 668, 67–75 (1995).
Li, F. and Cooper, S. F., The simultaneous identification of metoprolol and its major acidic and basic metabolites in human urine by gas chromatography-mass spectrometry.Instrum. Sci. Technol., 24, 13–22 (1996).
Murthy, S. S., Shetty, H. U., Nelson, W. L., Jackson, P. R. and Lennard, M. S., Enantioselective and diastereoselective aspects of the oxidative metabolism of metoprolol.Biochem. Pharmacol., 40, 1637–1644 (1990).
Nathanson, J. A., Stereospecificity of beta-adrenergic antagonists: R-enantiomers show increased selectivity for beta-2 receptors in the ciliary process.J. Pharmacol. Exp. Ther., 245, 94–101 (1988).
Pharm-Huy, C., Radenen, B., Sahui-Gnassi, A., and Claude, J., High-performance liquid chromatographic determination of (S)- and (R)-propanolol in human plasma and urine with a chiral β-cyclodextrin bonded phase.J. Chromatogr. B, 665, 125–132 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, K.H., Shin, S.D., Lee, J.H. et al. Chiral separation of the enantiomers of metoprolol and its metabolites by high performance liquid chromatography. Arch Pharm Res 23, 230–236 (2000). https://doi.org/10.1007/BF02976450
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02976450