Abstract
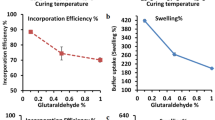
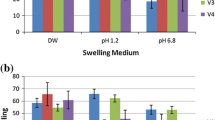
The sustained release dosage form which delivers melatonin (MT) in a circadian fashion over 8 h is of clinical value for those who have disordered circadian rhythms because of its short half-life. The purpose of this study was to evaluate the gelling properties and release characteristics of alginate beads varying multivalent cationic species (Al+++, Ba++, Ca++, Mg++, Fe+++, Zn++). The surface morphologies of Ca- and Ba-alginate beads were also studied using scanning electron microscope (SEM). MT, an indole amide pineal hormone was used as a model drug. The Ca++, Ba++, Zn++, Al+++, and Fe+++ ions except Mg++ induced gelling of sodium alginate. The strength of multivalent cationic alginate beads was as follows: Al+++≪Fe+++<Zn++<Ca++⋟Ba++. In case of Al+++, the induced hydrogel beads were very fragile and less spherical. Fe-alginate beads were also fragile but stronger compared to Al-alginate beads. Ba-alginate beads, had a similar gelling strength but was less spherical when compared to Ca-alginate beads. Zn-alginate beads were weaker than Ca- and Ba-alginate beads. Very crude and rough crystals of Ba- and Ca-alginate beads at higher magnifications were observed. However, the type and shape of rough crystals of Ba- and Ca-alginate beads were quite different. No significant differences in release profiles from MT-loaded multivalent cationic alginate beads were observed in the gastric fluid. Most drugs were continuously released upto 80% for 5 h, mainly governed by the passive diffusion without swelling and disintegrating the alginate beads. In the intestinal fluid, there was a significant difference in the release profiles of MT-loaded multivalent cationic alginate beads. The release rate of Ca-alginate beads was faster when compared to other multivalent cationic alginate beads and was completed for 3 h. Ba-alginate beads had a very long lag time (7 h) and then rapidly released thereafter. MT was continuously released from Fe-and Zn-alginate beads with initial burstout release. It is assumed that the different release profiles of multivalent cationic alginate beads resulted from forces of swelling and disintegration of alginate beads in addition to passive diffusion, depending on types of multivalent ions, gelling strength and drug solubility. It was estimated that 0.2 M CaCl2 concentration was optimal in terms of trapping efficiency of MT and gelling strength of Ca-alginate beads. In the gastric fluid, Ca-alginate beads gelled at 0.2 M CaCl2 concentration had higher bead strength, resulting in the most retarded release when compared to other concentrations. In the intestinal fluid, the decreased release of Ca-alginate beads prepared at 0.2 M CaCl2 concentration was also observed. However, release profiles of Ca-alginate beads were quite similar regardless of CaCl2 concentration. Either too low or high CaCl2 concentrations may not be useful for gelling and curing of alginate beads. Optimal CaCl2, concentrations must be decided in terms of trapping efficiency and release profiles of drug followed by curing time and gelling strength of alginate beads.
Similar content being viewed by others
References Cited
Benes, L., Brun, J., Claustrat, B., Degrande, G., Ducloux, N., Geoffriau, M., Horriere, F., Karsenty, H. and Lagain, D., Plasma melatonin (M) and Sulfatoxymelatonin (aMT6s) kinetics after transmucosal administration to humans. Melatonin and the pineal gland-from basic science to clinical application.Proceedings from the congress on pineal gland, Paris, France March, 347–350 (1993).
Bodmeier, R. and Paeratakul, O., Spherical agglomerates of water-insoluble drugs.J. Pharm. Sci., 78, 964–967 (1989).
Davies, N. M., Farr, S. J., Kellaway, I. W. and Thomas, G. T. M., A comparison of the gastric retention of alginate containing tablet formulations with and without the inclusion of excipient calcium ions.Int. J. Pharm., 105, 97–101 (1994).
Hwang, S-J., Rhee, G.-J., Jo, H.-B., Lee, K.-M. and Kim, C-K., Alginate beads as controlled release polymeric drug delivery system.J. Kor. Pharm. Sci., 23, 19–26 (1993).
Hwang, S-J., Rhee, G.-J., Lee, K.-M., Oh, K.-H. and Kim, C.-K., Release characteristics of ibuprofen from excipient-loaded alginate gel beads.Int. J. Pharm., 116, 125–128 (1995).
Kim, C.-K. and Lee, E.-J., The controlled release of blue dextran from alginate beads.Int. J. Pharm., 79, 11–19 (1992).
Koji, D., Yutaka, W., Chiaki, Y., Mamabu, Y., Seiji, O., Masayuki, O. and Takashi, M., Pharmacological studies of sodium alginate. I. Protective effect of sodium alginate on mucous membranes of upper-gastrointestinal tract.Yakugaku Zasshi, 101, 452–457 (1981).
Lee, B.-J. and Min, G-H., Preparation and release characteristics of polymer-reinforced and coated alginate beads.Arch. Pharm. Res., 18, 183–188 (1995).
Lee, B.-J. and Lee, J.-R., Enhancement of solubility and dissolution rate of poorly water-soluble naproxen by complexation with 2-hydroxypropyl-β-cyclodextrin.Arch. Pharm. Res., 18, 22–26 (1995).
Lee, B.-J., Parrott, K. A., Ayres, J. W. and Sack, R. L., Design and evaluation of an oral controlled release delivery system for melatonin in human subjects.Int. J. Pharm., 124, 119–127 (1995).
Lee, B.-J., Parrott, K. A., Ayres, J. W. and Sack, R. L., Preliminary evaluation of transdermal delivery of melatonin in human subjects.Res. comm. Mol. Pathol. & Pharmacol. 85, 337–344 (1994).
Ostberg, T., Vesterhus, L. and Graffner, C., Calcium alginate matrices for oral multiple unit administration: II. Effect of process and formulation factors on matrix properties.Int. J. Pharm., 97, 183–193 (1993).
Ostberg, T., Lun, E-M. and Graffner, C., Calcium alginate matrices for oral multiple unit administration: IV. Release characteristics in different media.Int. J. Pharm., 112, 241–248 (1994).
Smith, T. J., Calcium alginate hydrogel as a matrix for enteric delivery of nucleic acids.Pharm. Tech., 26–30 (1994).
Sugawara, S., Imai, T. and Otagiri, M., The controlled release of prednisolone using alginate gel.Pharm. Res., 11, 272–277 (1994).
Wee, S. and Gombotz, W., Controlled release of recombinant human tumor necrosis factor receptor from alginate beads.Proceed. Int. Symp. Contr. Rel. Bioact. Mater., 21, 730–731 (1994).
Yotsuyanagi, T., Ohkubo, T., Ohhashi, T. and Ikeda, K., Calcium-induced gelation of alginic acid and pH-sensitive reswelling of dried gels.Chem. Pharm. Bull., 35, 1555–1563 (1987).
Yuk, S. H., Cho, S. H. and Lee, H. B., pH-sensitive drug delivery system using O/W emulsion.J. Contr. Rel., 37, 69–74 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, BJ., Min, GH. & Kim, TW. Preparation andin vitro release of melatonin-loaded multivalent cationic alginate beads. Arch. Pharm. Res. 19, 280–285 (1996). https://doi.org/10.1007/BF02976241
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02976241