Abstract
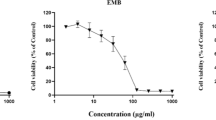
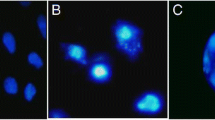
The detection of many synthetic chemicals used in industry that may pose a genetic hazard in our environment is subject of great concern at present. In this respect, the genetic toxicity of fenpropathrin ((RS)-α-cyano-3-phenoxybenzyl-2,2,3,3-tetramethyl cyclopropane carboxylate, CAS No.: 39515-41-8), a pyrethroid insecticide, was evaluated in bacterial gene mutation system, chromosome aberration in mammalian cell system andin vivo micronucleus assay with rodents. In bacterial gene mutation assay, no mutagenicity of fenpropathrin (62–5000 μg/plate) was observed inSalmonella typhimurium TA 98, 100, 1535 and 1537 both in the absence and in the presence of S-9 metabolic activation system. In mammalian cell system using chinese hamster lung fibroblast, no clastogenicity of fenpropathrin was also observed both in the absence andin the presence of metabolic activation system in the concentration range of 7–28 μg/ml. And also,in vivo micronucleus assay using mouse bone marrow cells, fenpropathrin also revealed no mutagenic potential in the dose range of 27–105 mg/kg body weight of fenpropathrin (i.p.). Consequently, no mutagenic potential of fenpropathrin was observedin vitro bacterial, mammalian mutagenicity systems andin vivo micronucleus assay in the dose ranges used in this experiment.
Similar content being viewed by others
References Cited
Abbo, S., Dunford, R. P., Miller, T. E., Reader, S. M. and King, I. P., Primer-mediatedin situ detection of the B-hordein gene cluster on barley chromosomes 1H.Genetics, 90, 11821–11824 (1993).
Altman, D. G.: Practical statistics for medical research. In Chapter 10: “Comparing groups-categorical data.” London: Chapman & Hall., 1993, pp. 229–276.
Ames, B. N., Durston, W. E., Yamasaki, E. and Lee, F. D., Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection.Proc. Natl. Acad. Sci. USA., 70, 2281–2285 (1973).
Ames, B. N., McCann, J. and Yamasaki, E., Method for detecting carcinogens and mutagens withSalmonella/mammalian-microsome mutagenicity test.Mutation Res., 31, 347–364 (1975).
Clive, D., McCuen, R., Spector, J. F. S., Piper, C. and Mavournin, K. H., Specific gene mutations in L 5178Y cells in culture: A report of the U.S. Environmental Protection Agency Gen-Tox Program.Mutation Res., 115, 225–251 (1983).
Hayashi, M., Sofuni, T. and Ishidate, M. Jr., High sensitivity in micronucleus induction of mouse strain.Mutation Res., 105, 252–256 (1982).
Hayashi, M., Morita, T., Kodama, Y., Sofuni, T. and Ishidate, M. Jr., The micronucleus assay with mouse peripheral blood reticulocytes using acridine organe-coated slides.Mutation Res., 245, 245–249 (1990).
Hayashi, M., Kodama, Y., Awogi, T., Suzuki, T., Asita, A. O. and Sofuni T., The micronucleus assay using peripheral blood reticulocytes from mitomycin C-and cyclophosphamide-treated rats.Mutation Res., 278, 209–213 (1992).
Hayashi, M., Maki-Paakkanen, J., Tanabe, H., Honma, M., Suzuki, T., Matsuoka, A., Mizusawa, H. and Sofuni, T., Isolation of micronuclei from mouse blood and fluorescence in situ hybridization with a mouse centromeric DNA probe.Mutation Res., 307, 245–251 (1994).
Ishidate, M. and Odashima, S., Chromosome test with 134 compounds on Chinese hamster cells in vitro-A screening for Chemical carcinogens.Mutation Res., 48, 337–354 (1977).
Jederny, J., Walk, R. A., Hackenberg, U. and Rohrborn, G., Chromosomal abnormalities and sister-chromatid exchange in bone marrow cells of mice and chinese hamsters after inhalation and intra-peritoneal administration.Mutation Res., 203, 1–10 (1988).
JEMS-MMS:Atlas of chromosome aberration by chemicals, Japanese Environmental Mutagen Society-Mammalian Mutagenicity Study Group. Tokyo, Japan (1988).
Kohler, S. W., Provost, G. S., Fieck, A., Kretz, P. L., Bullock, W. O., Sorge, J. A., Putman, D. L. and Short, J. M., Spectra of spontaneous and mutagen-induced mutations in thelac I gene in transgenic mice.Proc. Natl. Acad. Sci. USA, 88, 7958–7962 (1991).
Maron, D. M. and Ames, B. N., Revised methods for theSalmonella mutagenicity test.Mutation Res., 113, 173–215 (1983).
Matsuoka, A., Hayashi, M. and Ishidate, M., Chromosomal aberration test on 29 chemicals combined with S-9 mixin vitro.Mutation Res., 66, 277–290 (1979).
McCann, J., Choi, E., Yamasaki, E. and Ames, B. N., Detection of carcinogens as mutagens in theSalmonella/microsome test: assay of 300 chemicals.Proc. Natl. Acad. Sci. USA., 72, 5135–5139 (1975).
Mersch-Sundermann, V. S., Kerekordes, S. and Mochayedi, S., Sources of variability of theEscherichia coli PQ 37 genotoxicity assay (SOS chromotest).Mutation Res., 252, 51–60 (1991).
Mckelvey-Martin, V. J., Green, M. H. L., Schemezer, P., Pool-Zobel, B. L., De Meo, M. P. and Collins, A., The single cell gel electrophoresis assay (comet assay): A european review.Mutation Res., 288, 47–63 (1993).
Meselson, M. and Russel, K., Comparison of carcinogenic and mutagenic potency, In H.H. Hiatt, J. D. Watson and J. A. Winstend (Eds.),Origin of Human Cancer. Cold Spring Harbor Laboratory, 1977, pp. 1473–1481.
MOE report,Toxicity Evaluation of Synthetic Chemicals (V), Ministry of Environment, Seoul, Korea, 1992, pp. 19–63.
MOE report,Toxicity Evaluation of Synthetic Chemicals (VII), Ministry of Environment, Seoul, Korea, 1993, pp. 53–128.
MOE report,Toxicity Evaluation of Synthetic Chemicals (VII), Ministry of Environment, Seoul, Korea, 1994, pp. 29–129.
MOE report,Toxicity Evaluation of Synthetic Chemicals (VIII), Ministry of Environment, Seoul, Korea, 1995, pp. 61–103.
Mosmann, T., Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays.J. Immunol. Methods, 65, 55–63 (1983).
OECD.,OECD giudeline for the testing of chemicals, Documents 473, Genetic toxicology: in vitro mammalian cytogenetic test, Organization for Economic Co-operation and Development, Paris, France (1993).
Radman, M., Jeggo, P. and Wagner, R., Chromosomal rearrangement and carcinogenesis.Mutation Res., 98, 249–264 (1982).
Ryu, J.-C., Lee, S., Kim, K.-R., Kim, M., Chang, I.-M. and Park, J., A study on the clastogenicity of trichothecene mycotoxins in Chinese hamster lung cells.Korean J. Toxicol., 9(1), 13–21 (1993a).
Ryu, J.-C., Kim, S.-H., Kim, K.-R., Lee, S., Kim, M., Jung, H.-K. and Park, J., A study on the antimutagenic effect of cinnamic acid derivatives inEscherichia coli PQ 37 (SOS Chromotest) (I).Environ. Mutagens & Carcinogens, 13(1), 8–17 (1993b).
Ryu, E.-K., Kim, K.-R., Lee, S., Park, J. and Ryu, J.-C., Evaluation of the genetic toxicity of synthetic chemicals, chromosomal aberration test with 28 compounds in Chinese hamster lung cellsin vitro, The Fall Conference of the Korean Envirty of Toxicology and the Korean Environmental Mutagen Society, pp. 119 (1994a).
Ryu, J.-C., Lee, S., Kim, K.-R. and Park, J., Evaluation of the genetic toxicity of synthetic chemicals (I). Chromosomal aberration test on Chinese hamster lung cellsin vitro.Environ. Mutagens & Carcinogens, 14(2), 138–144 (1994b).
Ryu, J.-C., Kim, K.-R., Ryu, E.-K., Kim, H.-J., Kwon, O.-S., Song, C.-E., Mar, W. and Chang, I.-M., Chromosomal aberration assay of taxol and 10-deacetyl baccatin III in Chinese hamster lung cellsin vitro.Environ. Mutagens & Carcinogens, 16(1), 6–12 (1996).
Sato, T., Chikazawa, K., Yamamori, H., Ose, Y., Nagase, H. and Kito, H., Evaluation of the SOS chromotest for the detection of antimutagens.Environ. Mol. Mutagenesis, 17, 258–263 (1991).
Sawyer, J., Moore, M. M., Clive, D. and Hozier, J., Cytogenetic characterization of the L5178Y TK+/− 3. 7.2C mouse lymphoma cell line.Mutation Res., 147, 243–253 (1985).
Schmid, W., The micronucleus test.Mutation Res., 31, 9–15 (1975).
Singh, P. N., Stephens, R. E. and Schneider, E. L., Modification of alkaline microgel electrophoresis for sensitive detection of DNA damage.Int. J. Radiation Biol., 66(1), 23–28 (1994).
Suzuki, T., Hayashi, M., Sofuni, T. and Myhr, B. C., The concomitant detection of gene mutation and micronucleus induction by mitomycin Cin vivo usinglac Z transgenic mice.Mutation Res., 285, 219–224 (1993).
WHO Scientific group, The evaluation and testing of drug for mutagenicity, principles and problems.WHO Tech. Rep. Ser., No. 482 (1971).
Zimmermann, F. K., Induction of mitotic gene conversion by mutagens.Mutation Res., 11, 327–337 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ryu, JC., Kim, KR., Kim, HJ. et al. Evaluation of the genetic toxicity of synthetic chemicals (II), a pyrethroid insecticide, fenpropathrin. Arch. Pharm. Res. 19, 251–257 (1996). https://doi.org/10.1007/BF02976235
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02976235