Abstract
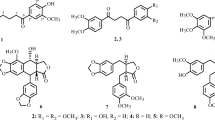
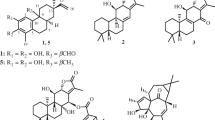
A cytotoxic constituent was isolated by bioassay-guided procedure from the roots ofSophora flavescens Aiton (Leguminosae). The constituent was identified as sophoraflavanone G (I) by means of chemical methods and in comparsion with spectral data of standard compound. The ED50 values of constituent I were 0.78, 1.57, 2.14 and 8.59 μg/ml against A549, HeLa, K562 and L1210 cell lines respectively. ConstituentI exhibited highly cytotoxic activities against A 549, K562 and HeLa cells, but showed a mild activity (ED50 value, 5 μg/ml) against L1210 cells. Among the tested cell lines, A549 cells were the most sensitive to constituentI.
Similar content being viewed by others
References Cited
Bae, K. H., Min, B. S., Do, D. S., Kim, N. S., Yang, K. J. and Ahn, B. Z., Screening on Cytotoxicity of Medicinal Plants against L1210 Cell.Yakhak Hoeji, 36, 491–495 (1992).
Bae, K. H., Min, B. S., Park, K. L. and Ahn, B. Z., Cytotoxic Flavonoids fromScutellaria indica.Planta Medica, 60, 280–281 (1994).
Ding, Y., Tian, R., Kinio, J., Nohara, T. and Kitagawa, J., Three new oleanene glycosides from Sophora flavescens.Chem. Pharm. Bull., 40, 2990–2994 (1992).
Kyogoku, K., Hatayama, K. and Komatsu, M., Constituents of Chinese crude drug “Kushen” (the root ofSophora flavescens Aiton.). Isolation of five new flavonoids and formononetic.Chem. Pharm. Bull., 21, 2733–2738 (1973).
Mabry, T. J., Markham, K. R. and Thomas, M. B.,The systematic identification of flavonoids, Spring-Verlag, New York, Chapter V. p. 41–226 (1970).
Min, B. S., Ahn, B. Z. and Bae, K. H., Snthesis and structure-activity relationship of cytotoxic 5,2′, 5′-trihydroxy-7,8-dimethoxyflavanone analogues.Arch. Pharm. Res., 19, 543–550 (1996).
Okuda, S., Murakoshi, I., Kamata, H., Kashida, Y., Haginiwa, J. and Tsuda, K., Studies on lupine alkaloids I. The minor alkaloids of JapaneseSophora flavescens.Chem. Pharm. Bull., 13, 482–487 (1965).
Perrin, D. D. and Armarego, W. L. F.,Purification of Laboratory Chemicals, 3rd ed., Pergamon, Oxford, 1988.
Saito, K., Arai, M., Sekine, T., Ohmiya, S., Kubo, H., Otomasu, H. and Murakoshi, I., (−)-5α-hydroxysophocarpine, a new lupine alkaloid from the seeds ofSophora flavescens var.angustifolia.Plant Medica., 56, 487–488 (1990).
Shirataki, Y., Endo, M., Yokoe, I. and Komatsu, M., Studies on the constituents ofSophora species XVIII. Constituents of the root ofSophora tomentosa L. (3).,Chem. Pharm. Bull., 31, 2859–2863 (1983).
Shirataki, Y., Yokoe, I., Noguchi, M., Tomimori, T. and Komatsu, M., Studies on the constituents of Sophora species XXII. Constituents of the root of Sophora moorcroftiag Benth. ex Baker (1).Chem. Pharm. Bull., 36, 2220–2225 (1988).
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J. T., Bokesch, H., Kenney, S. and Boyd, M. R., New colorimetric cytotoxicity for anticancer-drug screening.J. Natl. Cancer Inst., 82, 1107–1113 (1990).
Thayer, P. S., Himmelfarb, P. and Watts, G. L., Cytotoxicity assay with L1210 Cellsin vitro. Comparison with L1210 Cellsin vitro and KB Cellsin vitro.Cancer Chemother. Rep. (part 2) 2, 1 (1971).
Ueno, A., Morinaga, K., Fukushima, S. and Okuda, S., Studies on Lupine alkaloids VII. (−)-Δ7-dehydrosophoramine.Chem. Pharm. Bull., 26, 1832–1836 (1978).
Wu, L. T., Miyase, T., Ueno, A., Kuroyanagi, M., Noro, T., Fukushima, S. and Sasaki, S., Studies on the constituents ofSophora flavescens Aiton V.,Yakugaku Zasshi, 106, 22–26 (1986).
Wu, L. T., Miyase, T., Ueno, A. and Kuroyanaga, M., Studies on the constituents ofSophora flavescens Aiton IV.Yakugaku Zasshi, 105, 1036–1039 (1985a).
Wu, L. T., Miyase, T., Ueno, A. and Kuroyanaga, M., Studies on the constituents ofSophora flavescens Aiton II.Chem. Pharm. Bull., 33, 3231–3236 (1985b).
Wu, L. T., Miyase, T., Ueno, A. and Kuroyanaga, M., Studies on the constituents ofSophora flavescens Aiton III.Yakugaku Zasshi, 105, 736–741 (1985c).
Yagi, A., Fukunaga, M., Okuzako, N., Mifuchi, I. and Kawamoto, F., Antifungal substances fromSophora flavescens.Shoyakugaku Zasshi, 43, 343–347 (1989).
Yamazaki, M., Arai, A., Suzuki, S. and Takechi, T., Protective effects of matrine and oxymatrine on stress ulcer in relation to there effects on the central nervous system.Yakugaku Zasshi, 104, 293–301 (1984).
Yamaki, M., Kashihara, M. and Takagi, S., Activity of Kushen compounds againstStaphylococcus aureus andStreptococcus mutants.Phytother. Res., 4, 235–236 (1990).
Yashikawa, M., Wang, H. K., Kayakiri, H., Taniyama, T. and Kitagawa, I., Saponin and sapogenol XI. Structure of sophoraflavoside I, a bisdesmoside of soyasapogenol B, fromSophorae Radix, the root of Sophora flavescens Aiton.Chem. Pharm. Bull., 33, 4267–4274 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, Y.K., Min, B.S. & Bae, K.H. A cytotoxic constituent fromSophora flavescens . Arch. Pharm. Res. 20, 342–345 (1997). https://doi.org/10.1007/BF02976197
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02976197