Abstract
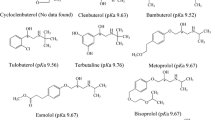
The β-blockers possess at least one chiral center and the S(-)-enantiomer shows higher affinity for binding to the β-adrenergic receptors than antipode. The stability constants of acebutolol, celiprolol, propranolol and terbutaline in the inclusion complexes with single-isomer heptakis (2,3-dimethyl-6-sulfato)-β-cyclodextrin (HDMS-β-CD) were determined by capillary electrophoresis. The approximation and linear double reciprocal methods were adapted with comparable results. Among the β-blockers studied, propranolol had the lowest stability constant but the highest enantioselectivity, indicating that the magnitudes of the stability constants carried little information about enantioseparation. The magnitudes of enantioselectivities between the enantiomer pair were in the order of propranolol > celiprolol > terbutaline > acebutolol.
Similar content being viewed by others
References
Bontchev, P. R., Kadum, H., Evtimova, B., Nachev, C., Ivanov, D., Zhecheva, E., and Mehandjiev, E., Copper(Il) complexes of (1-[isopropylamine]-3-[1-naphthyloxy]-2-propanol) (propranolol).J. Inorg. Biochem., 48, 153–161 (1992).
Borchard, U., Pharmacological prosperities of β-adrenoceptor blocking drugs.J. Clin. Bas. Cardiol., 1, 5–9 (1988).
Chankvetadze, B., Capillary electrophoresis in chiral analysis. John Wiley & Son, pp. 145–146 (1997).
Kim, K. H. and Park, Y. H., Enantioselective inclusion between terbutaline enantiomers and hydroxypropyl-β-cyclodextrin.Int. J. Pharm., 175, 247–253 (1998).
Mehvar R. and Brocks, D. R., Stereospecific pharmacokinetics and pharmacodynamics of β-adrenergic blockers in humans.J. Pharm. Pharmaceut. Sci., 4, 185–200 (2001).
Park, K. L., Kim, K. H., Jung, S. H., Lim, H. M., Hong, C. H., and Kang, J. S., Enantioselective stabilization of inclusion complexes of metoprolol in carboxymethylated β-cyclodextrin.J. Pharm. Biomed. Anal., 27, 569–576 (2002).
Park, K. R., Lim, H. M., Phuong, N. T., Kim, K. H., and Kang, J. S., Determination of stability constants for β-blocker and carboxymethyl-β-cyclodextrin complexes by capillary electrophoresis.Yakhak Hoeji, 47, 200–205 (2003).
Rundlett, K. L. and Arms, D. W., Examination of the origin, variation, and proper use of expression for the estimation of association constants by capillary electrophoresis.J. Chromatogr. A, 721, 173–186 (1996).
Stoschizly, K., Zernig, G., and Lindner, W., Racemic β-blockers-fixed combinations of different drugs.J. Clin. Bas. Cardiol., 1, 14–18 (1998).
Toda, N., Vasodilating β-adrenoceptor blockers as cardiovascular therapeutics.Pharm. Therapeu., 100, 215–234 (2003).
Vargas, M. G., Heyden, Y. V., Maftouh, M., and Massart, D. L., Rapid development of the enantiomeric separation of β-blockers by capillary electrophoresis using an experimental design approach.J. Chromatogr. A, 855, 681–693 (1999).
Vespalec, R. and Bocek, P., Calculation of stability constants for the chiral selector-enantiomer interaction from electrophoretic mobilities.J. Chromatogr. A, 875, 431–445 (2000).
Wikstrand, J., Berglund, G., Hedblad, B., and Hulthe, J., Antiatherosclerotic effects of β-blockers.Am. J. Cardiol., 12, 25–29 (2003).
Williams, B. A. and Vigh, G., Dry look at the CHARM (charge resolving agent migration) model of enantiomer separations by capillary electrophoresis.J. Chromatogr. A, 777, 295–309 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phuong, N.T., Lee, K.A., Kim, K.H. et al. Determination of stability constants of the inclusion complexes of β-blockers in heptakis (2,3-dimethyl-6-sulfato)-β-cyclodextrin. Arch Pharm Res 27, 1290–1294 (2004). https://doi.org/10.1007/BF02975896
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02975896