Abstract
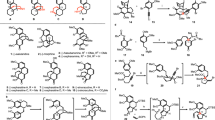
A functional divergency oriented synthetic approach to the azoninone (9-membered lactams), key intermediate for the indolizidine alkaloids library, using amide enolate induced aza-Claisen rearrangement has been achieved.
Similar content being viewed by others
References
Burke, M. D. and Schreiber, S. L., A planning strategy for diversity-oriented synthesis.Angew. Chem., Int. Ed. Engl., 43, 46–58 (2004).
Castro, A. M. M., Claisen rearrangement over the past nine decades.Chem. Rev., 104, 2939–3002 (2004).
Coates, R. M., Rogers, B. D., Hobbs, S. J., Curran, D. P., and Peck, D. R., Synthesis and Claisen rearrangement of alkoxyallyl enol ethers. Evidence for a dipolar transition state.J. Am. Chem. Soc., 109, 1160–1170 (1987).
Diederich, M. and Nubbemeyer, U., Synthesis of optically-active 9-membered ring lactams by a zwitterionic, aza-Claisen reaction.Angew. Chem., Int. Ed. Engl., 34, 1026–1028 (1995).
Edstrom, E. D., New methodology for the synthesis of functionalized indolizidine and quinolizidine ring systems.J. Am. Chem. Soc., 113, 6690–6692 (1991).
Evans, P. A. and Holmes, A. B., Medium ring nitrogen heterocyles.Tetrahedron, 47, 9131–9166 (1991).
Guengerich, F. P. and Bronquist, H. P., InBioorganic Chemistry, Ed. Van Tameleon, E. E. Academic, New York, Vol 2, 97–109 (1979).
Michael, J. P., Indolizidine and quinolizidine alkaloids.Nat. prod. Rep., 18, 520–542 (2001).
Molyneux, R. J., Tropea, J. E., and Elbein, A. D., 7-Deoxy-6-epi- castanospermine, a trihydroxyindolizidine alkaloid glycosidase inhibitor from Castanospermum australe.J. Nat. Prod., 53, 609–614 (1990).
Nubbemeyer, U., Synthesis of medium-sized ring lactams.Top. Curr. Chem., 216, 125–196 (2001).
Pereira, S. and Srebnik, M., The Ireland-Claisen rearrangement.Aldrichimica Acta, 26, 17–29 (1993)
Sudau, A. and Nubbemeyer, U., Unusual diastereoselection in the synthesis of 9-membered ring lactams and conformation-controlled transannular reactions to generate optically active indolizidinones.Angew. Chem. Int. Ed. Engl., 37, 1140–1143 (1998).
Suh, Y.-G., Kim, S.-A., Jung, J.-K., Shin, D.-Y., Min, K.-H., Koo, B.-A., and Kim, H.-S., Asymmetric Total Synthesis of Fluvirucinine A1.Angew. Chem. Int. Ed. Engl., 38, 3545–3547 (1999).
Suh, Y.-G., Lee, J.-Y., Kim, S.-A., and Jung, J.-K., A new ring expansion reaction of 1-acyl-2-vinylpiperidine and 1-acyl-2- vinylpiperazinevia aza-Claicen rearrangement of amide enolate.Synth. Commun., 26, 1675–1680 (1996).
Tsunoda, T., Tatsuki, S., Shiraishi, Y., Akasaka, M., and Ito, S., Asymmetric aza-Claisen rearrangement of glycolamide and glycinamide enolates. Synthesis of optically active a-hydroxy and a-amino acids.Tetrahedron Lett., 34, 3297–3300 (1993).
Vedejs, E. and Gingras, M., Aza-Claisen rearrangements initiated by acid-catalyzed Michael addition.J. Am. Chem. Soc., 116, 579–588 (1994).
Yet, L., Metal-mediated synthesis of medium-sized rings.Chem. Rev., 100, 2963–3007 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/BF02975148.
Rights and permissions
About this article
Cite this article
Jung, J.K., Choi, N.S. & Suh, Y.G. Functional divergency oriented synthesis of azoninones as the key intermediates for bioactive indolizidine alkaloids analogs. Arch Pharm Res 27, 985–989 (2004). https://doi.org/10.1007/BF02975418
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02975418