Abstract
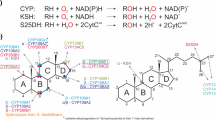
Steroid 9α-hydroxylase is an enzyme found in nocardioform microorganisms which can utilize steroids as a sole carbon source. After fractional centrifugation of the cell homogenates, the enzyme activity inNocardia andRhodococcus was found in cytoplasmic membrane fraction. On the contrary,Mycobacterium had its 9α-hydroxylation activity in cytosolic fraction. To characterize the enzyme in these microorganisms, several potential inhibitors of 9α-hydroxylase were tested and the cofactor requirement for the same enzyme was also examined. The inhibitory effect of ferrous ion chelators indicated involvement of iron containing proteins in the 9α-hydroxylase system. On the other hand, metyrapone, an inhibitor known to be specific for cytochrome P450 interfered with the enzyme inMycobacterium, but didn’t inhibit the enzyme activity inNocardia andRhodococcus. While the 9α-hydroxylase system inNocardia andRhodococcus required NADPH, NADH was required as an election donor inMycobacterium.
Similar content being viewed by others
References Cited
Asperger, O., Naumann, A. and Kleber, H. P., Inducibility of cytochrome P450 inAcinetobacter calcoaceticus by n-alkanes.Appl. Microbiol. Biotechnol., 19, 398–403 (1984).
Berg, A., Gustafsson, J. A. and Ingelman-Sundberg, M., Characterization of a cytochrome P450-dependent steroid hydroxylase system present inBacillus megaterium.J. Biol. Chem., 251, 2831–2838 (1976).
Berg, A., Ingelman-Sundberg, M. and Gustafsson, J. A., Purification and characterization of cytochrome P450meg.J. Biol. Chem., 254, 5264–5271 (1979).
Bernhardt, F. H., Pachowsky, H. and Staudinger, H., A 4-methoxybenzoateo-demethylase fromPseudomonas putida, a new type of monooxygenase system.Eur. J. Biochem., 57, 241–256 (1975).
Bradford, M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal. Biochem., 72, 248–254 (1976).
Bradford, O. F., Adaptation of the Bradford protein assay to membrane-bound proteins by solubilizing in glucopyranoside detergents.Anal. Biochem. 162, 11–17 (1987).
Chang, F. N. and Sih, C. J., Mechanisms of steroid oxidation by microorganisms. VII. Properties of the 9α-hydroxylase.Biochem., 3, 1551–1557 (1964).
De Frank, J. J. and Ribbons, D. W., p-Cymene pathway inPseudomonas putida: initial reactions.J. Bacteriol., 129, 1356–1364 (1977).
Dodson, R. M. and Muir, R. D., Microbiological transformations: VII The hydroxylation of steroids at C-9.J. Am. Chem. Soc., 83, 4631–4635 (1961).
Ericson, A., Hedman, B., Hodgson, K. O., Green, J., Dalton, H., Bentsen, J. G., Beer, R. H. and Lippard, S. J., Structural characterization by EXAFS spectroscopy of the binuclear iron center in Protein A of methane monooxygenase fromMethylococcus capsulatus (Bath).J. Am. Chem. Soc., 110, 2330–2332 (1988).
Fox, B. G., Liu, Y., Dege, J. E. and Lipscomb, J. D., Complex formation between the protein components of methane monooxygenase fromMethylosinus trichosporium OB 3b.J. Biol. Chem., 266, 540–550 (1991).
Frans, J. W., Willen, J. H. Van Berkel, Hartmans, S. and Jan, A. M. De Bont., Purification and properties of the NADH reductase component alkene monooxygenase fromMycobacterium strain E3.J. Bacteriol., 174, 3275–3281 (1992).
Hartmans, S., Frans, J. W., Somhorst, D. P. M. and Jan A. M. De Bont., Alkene monooxygenase fromMycobacterium: a multicomponent enzyme.J. Gen. Microbiol., 137, 2555–2560 (1991).
Ho, P. P. and Fulco, A. J., Involvement of a single hydroxylase species in the hydroxylation of palmitate at the ω-1, ω-2, and ω-3 positions by a preparation fromBacillus megaterium.Biochim. Biophys. Acta, 431, 249–256 (1976).
Itagaki, E., Studies on steroid monooxygenase fromCylindrocarpon radicicola ATCC 11011; Purification and characterization.J. Biochem., 99, 815–824 (1986).
Katagiri, M., Ganguli, B. N. and Gunsalus, I. C., A soluble cytochrome P450 functional role in methylene hydroxylation.J. Biol. Chem., 243, 3543–3546 (1968).
O’Keffe, D. P., Romesser, J. A. and Leto, K. J., Identification of constitutive and herbicide inducible cytochromes P450 in Streptomyces griseolus.Arch. Microbiol., 149, 406–412 (1988).
Peterson, J. A., Basu, D. and Coon, M. J., Fatty acid ω-hydroxylating system ofPseudomonas oleovorans.J. Biol. Chem. 241, 5162–5164 (1966).
Prior, S. D. and Dalton, H., The effect of copper ions on membrane content and methane monooxygenase activity in methanol-grown cells ofMethylococcus capsulatus (Bath).J. Gen. Microbiol., 131, 155–163 (1985).
Ramachandra, M. and Sariaslani, F. S., Identification of a ferredoxin reductase in cell-free extracts ofStreptomyces griseus. Abstr. 90th Ann. Melt.Am. Soc. Microbiol. Abstr., 0–54 p. 273 (1990).
Sebek, O. K. and Perlman, D.; Microbial Transformation of Steroids and Sterols, In Peppler, H. J. and Perlman, D. (Eds.).Microbial technology, 2nd Ed.; Academic Press, New York. 1, pp. 483–496, 1979.
Stanley, S. H., Prior, S. D., Leak, D. J. and Dalton, H., Copper stress underlies the fundamental change in intracellular location of methane monooxygenase in methane-oxidizing organisms: Studies in batch and continuous cultures.Biotech. Lett., 5, 487–492 (1983).
Strijewski, A., The steroid 9α-hydroxylation system fromNocardia species.Eur. J. Biochem., 128, 125–135 (1982).
Trower, M. K., Sariaslani, F. S. and O’keefe, P. O., Purification and characterization of a soybean flour-induced cytochrome P450 fromStreptomyces griseus.J. Bacteriol., 171, 1781–1787 (1989).
Ullah, A. J. H., Murray, R. I., Bhattacharyya, P. K., Wagner, G. C. and Gunsalus, I. C., Protein components of a cytochrome P450 linalool 8-methyl hydroxylase.J. Biol. Chem., 265, 1345–1351 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kang, H.K., Lee, S.S. Heterogeneous natures of the microbial steroid 9α-hydroxylase in nocardioforms. Arch. Pharm. Res. 20, 519–524 (1997). https://doi.org/10.1007/BF02975204
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02975204