Abstract
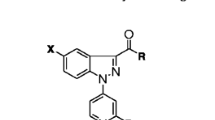
2,3-Diphenyl-6-formyl-5-methoxyindole reacts with ethyl cyano acetate to yield the arylidene derivative which forms with urea and thiourea the corresponding pyrimidine derivatives. The arylidene derivatives react with hydrazines and with active methylenes to form the respective pyrazole derivatives and the α, β-disubstituted acrylonitriles. Seven new compounds were tested for their effects on the arterial blood pressure of rats and analgesic activity.
Similar content being viewed by others
Literature Cited
Hori, M., Ito, E., Takita, T., Koyama, G., Takeuchi, T. and Umezawa, H.: A new antibiotic formycin.J. Antibiotics (Tokyo), Ser. A.17, 96 (1964).
Kayama, G. and Umezawa, H.: Formycin B and its relation to Formycin.J. Antibiotics (Tokyo), Ser. A.18, 175 (1965).
Szmuszkovicz, J.: Antiinflammatory, antipyretic and analgestic trifluoromethyl substituted 2,3-bis (p-methoxyphenyl) indoles. U. S. 3,551,451 (Cl. 260.326.16; (07d), 29 Dec. 1970, Appli. 26 May (1967); 4 pp.
Szmuszkovicz, J.: Antiinflammatory 5-alkonyl-2, 3-bis (p-methoxyphenyl) indoles. U. S. 3,565,912 (Cl. 260-326.16; (07d), 23 Feb. 1971. Appl. 27 Jan. (1969); 8 pp.
Szmuszkovicz, J.: Antiinflammatory 2,3-bis (p-methoxyphenyl) indole 5-carboxylic acid derivatives. U. S. 3,654,308 (Cl. 260–326. 13R; (07d) 04 Apr. 1972. Appl. 479,402,27 Jan (1968); 8 pp.
Whitehead, C. W. and Whitesitt, C. A.: Effect of lipophilic substituents on some biological properties of indoles.J. Med. Chem. 17, 1298 (1974).
Ockenden, D. W. and Schoffied, K.: Indoles part III. The action of (A) ozone and (B) osmium tetroxide on some indole derivatives.J. Chem. Soc. 612 (1953).
Lundt, B. F., and Anderson, W.: Analgesic 3-(aminoalkoxy) indoles.Ger. Offen. 2,024,966 (Cl. C. 07d), 03 Dec. 1970,Brit. Appl. 27 (1969), 42 pp.
Mcleod, L. J.: “Pharmacological experiments on intact preparations”, E & S, Livingstone Edindurgh and London (1970).
Jansen, P. A. J. and Jageneau, A. H.: “Series of potent analgesics-d-2,2-diphenyhl-3-methyl-4-morpholino butyl pyrolidine and related basic amides. (II) Comparative analgesic activity, acute toxicity and tolerance development in rats for R, 875, morphine, pethidine and methadone”,J. Pharm. Pharmac. 9, 381 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hishmat, O.H., Nakkady, S.S., El Shabrawy, O.A. et al. Synthesis and biological activities of new substituted indoles. Arch. Pharm. Res. 15, 104–108 (1992). https://doi.org/10.1007/BF02973994
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02973994