Abstract
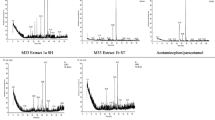
In order to obtain acetaminophen, a popular analgesic-antipyretic, though microbial p-hydroxylation and N-acetylation of aniline, variousStreptomyces strains were screened. Aniline N-acetylation activity was rather ubiquitous but p-hydroxylation activity was selective. Microbial conversion pathway of aniline to acetaminophen was considered to be through N-acetylation and p-hydroxylation orvice versa. However, depending on species used, o-hydroxylation and its degradation activity (S. fradiae) and acetaminophen degradation activity (S. coelicolar) were also detected. Among the screenedStreptomyces strains.S. fradiae NRRL 2702 showed the highest acetanilide p-hydroxylation activity (2–3% conversion rate). Furthermore, inS. fradiae carbon source and its concentration, phosphate ion concentration and pH of growth medium were found to play the crucial roles in p-hydroxylation activity. Through the proper combination of factors mentioned above, the ten times more activity (26–30% conversion rate) was attained.
Similar content being viewed by others
Literature Cited
Gibson, D. T.: Microbial degradation of aromatic compounds.Science 161, 1093 (1968).
Ferris, J. P., Fasco, M. J., Stylianopoulou, F. L., Jerina, D. M., Daily, J. W. and Jeffrey, A. M.: Monooxygenase activity inCunninghamella bainieri: Evidence for a fungal system similar to liver microsomes.Arch. Biochem. Biophys. 156, 97 (1973).
Berg, A. and Rafter, J.: Studies on the substrate specificity and inducibility of cytochrome P-450meg.Biochem. J.,196, 781 (1981).
Smith, R. V. and Rosazza, J. P.: Microbial models of mammalian metabolism. Aromatic hydroxylation.Arch. Biochem. Biophys. 161, 551 (1974).
Sohn, Y. W. and Lee, S. S.: Microbial synthesis of acetaminophen.Seoul Univ. J. Pharm. Sci. 12 (1987).
Theriault, R. J. and Longfield: Microbial conversion of acetanilide to 2-hydroxyacetanilide and 4-hydroxyacetanilide.Appl. Microbiol. 67, 1431 (1967).
Demain, A. L.: Catabolic regulation in industrial microbiology inOverproduction of microbial products. FEMS symposium ed. Krumphanzl, V., Sikyta, B. and Banek, Z., Academic Press Inc., New York, pp. 3 (1982).
Fulco, A. J.: P450BM-3 and other inducible bacterial P450 cytochromes: Biochemistry and regulation.Ann. Rev. Pharmacol. Toxicol. 31, 177 (1991).
Okey, A. B.: Enzyme induction in the cytochrome P-450 system.Pharmacol. Ther. 45, 241 (1990).
Sariaslani, F. S. and Kunz, D. A.: Induction of cytochrome P-450 inStreptomyces griseus soybean flour.Biochem. Biopophys. Res. Commun. 141, 405 (1986).
Fulco, A. J. and Ruettinger, R. T.: Occurrence of a barbiturate-inductible catalytically self-sufficient 119,000 dalton cytochrome P-450 monooxygenase inBacilli.Life Sic. 40, 1769 (1987).
Hathway, D. E.: Plant phenols and tannins. InChromatographic and electrophoretic techniques. vol. 1 ed. Smith, I., Wm. Heinemann Medical Book Ltd., London, p. 324 (1960).
Shihabi, Z. K. and David, R. M.: Colorimetric assay for acetaminophen in serum.Ther. Drug Monitor. 6, 449 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jin, H.J., Park, A.K. & Lee, S.S. Bioconversion of aniline to acetaminophen and overproduction of acetaminophen byStreptomyces spp.. Arch. Pharm. Res. 15, 41–47 (1992). https://doi.org/10.1007/BF02973982
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02973982