Abstract
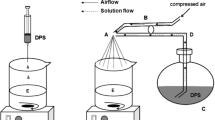
Prolonged release microparticles of clarithromycin (CL) were prepared using EudragitRL 100 andRS 100 by spray-drying and casting-drying techniques. For the characterization of those microparticles, preparation yield, particle size distribution, X-ray diffraction, thermal behavior, active agent content andin vitro dissolution from the microparticles were performed. HPLC was used for the assay of clarithromycin and the assay method was validated. All the formulations obtained showed prolonged release when compared to pure clarithromycin. Microparticles prepared by spray-drying method had a slower release compared to those of casting-drying method. Spray-drying method seems to be a more suitable method to prepare microparticles for prolongation in release.
Similar content being viewed by others
References
Adam, D., Glaser-Caldow, E., Wachter, J., Brueckner, O.-J., Hein, J., Kroemer, B., and Hirsch, J., Comparative efficacy of clarithromycin modified rlelase and immediate relaese formulations in the treatment of lower respiratory tract infection.Clin. Ther., 23(4), 585–595 (2001).
Barradell, L. B., Plosker, G. L., and Mctavish, D., Clarithromycin: A review of its pharmacological properties and therapeutic use inMycobacterium avium-intracellulare complex infection in patients with Acquired Immune Deficiency Syndrome.Drugs, 46(2), 289–312 (1993).
Bele, M., Dmitrasinovic, D., Planinsek, O., Salobir, M., Srcic, S., Gabescek, M., and Jamnik, J., Silica coating on clarithromycin.Int. J. Pharm., 291, 149–153 (2005).
Beuving, G., Validation of Analytical Methods. Validation Seminar, Istanbul, 31 May–01 June 2001.
Chu, S.-Y., Sennello, L. T., and Sonders, R. C., Simultaneous determination of clarithromycin and 14-hydroxy-clarithromycin in plasma and urine using HPLC with electronical detection.J. Chromatogr., 571, 199–208 (1991).
Conte, J. E., Golden, J. A., Duncan, S., Mckenna, E., and Zurlinden, E., Intra-pulmonar pharmacokinetics of clarithromycin and of erythromycin.Antimicrob. Agents Chemother., 39(2), 334–338 (1995).
Erah, P. O., Goddard, A. F., Barrett, D. A., Shaw, P. N., and Spillr, R. C., The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment ofHelicobacter pylori infection.J. Antimicrob. Chemother., 39, 5–12 (1997).
Guay, D. R. P., Gustavson, L. E., Devcich, K. J., Zhang, J., Cao, G., and Olson, C. A., Pharmacokinetics and tolerability of extended-release clarithromycin.Clin. Ther., 23(4), 566–577 (2001).
Kim, S. J., Hahn, S. K., Kim, M. J., Kim, D. H., and Lee, Y. P., Development of a novel sustained release formulation of recombinant human growth hormone using sodium hyaluronate microparticles.J. Control. Release, 104, 323–335 (2005).
Li, Y., Zhu, K. J., Zhang, J. X., Jiang, H. L., Liu, J. H., Hao, Y. L., Yasuda, H., Ichimaru, A., and Yamamoto, K., In vitro and in vivo studies of cyclosporin A-Loaded microspheres based on copolymers of lactide and ε-Caprolactone: Comparison with Conventional PLGA Microspheres.Int. J. Pharm., 295, 67–76 (2005).
Louis, St., Drug Facts and Comparisons. A Wolters Kluwer Company, USA, p. 2189 (1997).
Martin, T. M., Bandi, N., Shulz, R., Roberts, C. B., and Kompella, U. B., Preparation of budesonide and budesonide-PLA microparticles using supercritical fluid precipitation technology.AAPS Pharm Sci. Tech., 3(3), 1–11 (2002).
Morgan, D. K., Brown, D. M., Rotsch, T. D., and Plasz, A. C., A reversed-phase HPLC method for the determination and identification of clarithromycin as the drug substance and in various dosage forms.J. Pharm. Biomed. Anal., 9(3), 261–269 (1991).
Mu, I., Teo, M.-M., Ning, H.-Z., and Feng, S.-S., Novel powder formulations for controlled delivery of poorly soluble anticancer drug: Application and investigation of TPGS and PEC in spray-dried particulate system.J. Control. Release, 103, 565–575 (2005).
Nakagawa, Y., Itai, S., Yoshida, T., and Nagai, T., Physicochemical properties and stability in the acidic solution of a new macrolide antibiotic, clarithromycin, in comparison with erythromycin.Chem. Pharm. Bull., 40(3), 725–728 (1992).
Oneda, F. and Ré, M. I., The effect of formulation variables on the dissolution and physical properties of spray-dried microspheres containing organic salts.Powder Technol., 130, 377–384 (2003).
Pav, B., Misztal, G., Hopkala, H., and Drozd, J., Development and validation of a HPLC method for the determination of cetrizine in pharmaceutical dosage forms.Pharmazie, 57, 313–315 (2002).
Perng, C. Y., Kearney, A. S., Patel, K., Palepu, N. R., and Zuber, G., Investigation of formulation approaches to improve the dissolution of SB-210661 a poorly water soluble 5-Lipoxy-genase inhibitor.Int. J. Pharm., 176, 31–38 (1998).
Reiley, C. M. and Fell, F., Development and validation of analytical methods.Prog. Pharm. Biomed. Anal., 3, 20–22 (1996).
Rotsch, T. D., Spanton, M., Cugier, P., and Plasz, A. C., Determination of clarithromycin as a conta-minant on surfaces by HPLC using electronical detection.Pharm. Res., 8(8), 989–991 (1991).
U. S. Pharmacopeia National Formulary, USP 26-NF 21, 3th Ed., Mack Printing Company Easton, U.S.A., pp. 2439–2445 (2003).
Yonemochi, E., Kitahara, S., Maeda, S., Yamamura, S., Oguchi, T., and Yamoto, K., Physicochemical properties of amorphous clarithromycin obtained by grinding and spray drying.Eur. J. Pharm. Sci., 7, 331–338 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Genç, L., Demirel, M. & Yazan, Y. Preparation of prolonged release clarithromycin microparticles for oral use and theirin vitro evaluation. Arch Pharm Res 29, 921–927 (2006). https://doi.org/10.1007/BF02973915
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02973915