Abstract
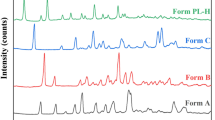
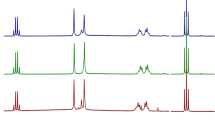
Nine polymorphic modifications of doxazosin mesylate have been obtained by recrystallization in organic solvents under variable conditions. Different polymorphs of doxazosin mesylate were characterized by powder X-ray crystallography diffractometry (PXRD), differential scanning calorimetry (DSC), and thermogravimetric analysis (TG). Transformation of Form 1 and Form 2 was not occurred in three relative humidities (0%, 51%, and 99%) at 20±0.5 for 30 days.
Similar content being viewed by others
References
Borka, L., Reviews on crystal polymorphism of substances in the European Pharmacopoeia.Pharm Acta Helv., 66, 16–22 (1991).
Giron, D., Thermal analysis and calorimetric methods in the characterization of polymorphs and solvates.Thermochim. Acta, 248, 1–59 (1995).
Grcman, M., Vrecer, F., and Meden, A., Some physico-chemical properties of Doxazosin mesylate polymorphic forms and its amorphous state.J. Them. Anal. Cal., 68, 373–387 (2002).
Gridhar, T., Reddy, R. B., and Ramesh, C., Methods for the preparation of polymorphs of doxazosin mesylate.US 6, 399, 775B1 (2002).
Gruenenberg, A., Polymorphie und thermische Analyse pharmazeutischer WirkstoffePharmazie in unserer Zeit, 26, 224–231 (1997).
Haleblian, J. and McCrowne, W., Pharmaceutical applications of polymorphism.J. Pharm. Sci., 58, 911–929 (1969).
Haleblian, J., Characterization, of habits and crystalline modification of solids and their pharmaceutical applications.J. Pharm. Sci., 65, 1269–1288 (1975).
Kuhnert-Brandstaetter, M., Polymorphie von Arzneitoffen und ihre Bedeutung in der pharmazeutischen Technologie.Informationsdienst A.P.V., 19, 73–90 (1973).
Sohn, Y. T., Effect of polymorphism on bioavailability of amoxicillin.Yakhak Hoeji, 39, 438–443 (1995).
Sohn, Y. T. and Kim, S. Y., Effect of crystal form onin vivo topical anti-inflammatory activity of corticosteroids.Arch. Pharm Res., 25, 556–559 (2002).
Sun, C. and Grant, D. J. W., Influence of crystal structure on the tableting properties of sulfamerazine polymorphs.Pharm. Res., 18, 274–280 (2001).
Yoshinari, T., Forbes, R. T., York, P., and Kawashima, Y., The improved compaction properties of mannitol after a moisture-induced polymorphic transition.Int. J. Pharm., 258, 121–131 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sohn, YT., Lee, YH. Polymorphism of doxazosin mesylate. Arch Pharm Res 28, 730–735 (2005). https://doi.org/10.1007/BF02969365
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02969365