Abstract
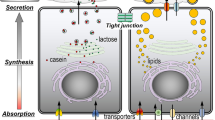
In the present study, a human mammary epithelial cell (HMEC) culture model was developed to evaluate the potential involvement of carrier-mediated transport systems in drug transfer into milk. Trypsin-resistant HMECs were seeded on Matrigel®-coated filters to develop monolayers of functionally differentiated HMEC. Expression of the specific function of HMEC monolayers was dependent of the number of trypsin treatments. Among the monolayers with different numbers of treatment (treated 1 to 3 times), the monolayer treated 3 times (3-t-HMEC monolayer) showed the highest maximal transeptithelial resistance and expression of β-casein mRNA as an index of differentiation. Transport of tetraethylammonium (TEA) across the 3-t-HMEC monolayer in the basolateral-to-apical direction was significantly higher than that in the apicalto-basolateral direction (p<0.05), whereas such directionality was not observed forp-amino-hippurate, suggesting the existence of organic cation transporters, but not organic anion transporters. In fact, expression of mRNAs of human organic cation transporter (OCT) 1 and 3 were detected in the 3-t-HMEC monolayer. These results indicate that the 3-t-HMEC monolayer is potentially useful for the evaluation of carrier-mediated secretion of drugs including organic cations into human milk.
Similar content being viewed by others
References
Adson, A., Raub, T. J., Burton, P. S., Barsuhn, C. L., Hilgers, A. R., Audus, K. L., and Ho, N. F., Quantitative approaches to delineate paracellular diffusion in cultured epithelial cell monolayers.J. Pharm. Sci., 83, 1529–1536 (1994).
Alcom, J., Lu, X., Moscow, J. A., and McNamara, P. J., Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction.J. Pharmacol. Exp. Ther., 303, 487–496 (2002).
American Academy of Pediatrics, Committee on Drugs, The transfer of drugs and other chemicals into human milk.Pediatrics, 108, 776–789 (2001).
American Academy of Pediatrics, Work Group on Breast-feeding, Breastfeeding and the use of human milk.Pediatrics, 100, 1035–39 (1997).
Anderson, P. O., Drug use during breastfeeding.Clin. Pharm., 10, 594–624 (1991).
Bennett, P. N., (ed), Drugs and Human Lactation, 2nd edition. Elsevier, Amsterdam (1996).
Berga, S. E., Electrical potentials and cell-to-cell dye movement in mouse mammary gland during lactaion.Am. J. Physiol., 247, C20-C25 (1984).
Fleishaker, J. C., Desai, N., and McNamara, P. J., Factors affecting the milk-to-plasma drug concentration ratio in lactating women: physical interactions with protein and fat.J. Pharm. Sci., 76, 189–193 (1987).
Fleishaker, J. C., Models and methods for predicting drug transfer into human milk.Adv. Drug Deliv. Rev., 55, 643–652 (2003).
Food and Drug Administration (US), 2005, Guidance for Industry, Clinical Lactation studies — study design, data analysis, and recommendations for labeling. Maryland, Available from: http://www.fda.gov/cder/guidance/index.htm.
Gerk, P. M., Kuhn, R. J., and McNamara, P. J., Active transport of nitrofurantoin into human milk.Pharmacotherapy, 21, 669–675 (2001).
Gerk, P. M., Moscow, J. A., and McNamara, P. J., Basolateral active uptake of nitrofurantoin in the CIT3 cell culture model of lactation.Drug Metab. Dispos., 31, 691–693 (2003).
Ito, S. and Lee, A., Drug excretion into breast milk overview.Adv. Drug Deliv. Rev., 55, 612–617 (2003).
Linzell, J. L. and Peaker, M., The permeability of mammary ducts.J. Physiol., 201, 701–716 (1971).
Linzell, J. L. and Peaker, M., Changes in colostrums composition and in the permeability of the mammary epithelial at about the time of parturition in the goat.J. Physiol., 243, 129–151 (1974).
Marks, G. J., Ryan, F. M., Hidalgo, I. J., and Smith, P. L., Mannitol as a marker for intestinal integrity inin vitro absorption studies.Gastroenterology 100, suppl, A697 (1991).
McManaman, J. L. and Neville, M. C., Mammary physiology and milk secretion.Adv. Drug Deliv. Rev., 55, 629–641 (2003).
McNamara, P. J., Burgio, D., and Yoo, S. D., Pharmacokinetics of cimetidine during lactation: species differences in cimetidine transport into rat and rabbit milk.J. Pharmacol. Exp. Ther., 261, 918–923 (1992).
Neville, M. C., Morton, J., and Umemura, S. Lactogenesis: the transition from pregnancy to lactation.Pediatr. Clin. North Am., 48, 35–52 (2001).
Oo, C. Y., Kuhn, R. J., Desai, N., and McNamara, P. J., Active transport of cimetidine into milk.Clin. Pharmacol Ther., 58, 548–555 (1995).
Schmidhauser, C., Bissell, M. J., and Myers, C. J., Casperson, F. G., Extracellular matrix and hormones transcriptionally regulate bovine â-casein 5′ sequences in stably transfected mouse mammary cells.Proc. Natl. Acad. Sci. U.S.A., 87, 9118–9122 (1990).
Shipman, L. J., Docherty, A. H., Knight, C. H., and Wilde, C. J., Metabolic adaptations in mouse mammary gland during a normal lactation cycle and in extend lactation.Q. J. Exp. Physiol., 72, 303–311 (1987).
Toddywalla, V. S., Kari, F. W., and Neville, M. C., Active transport of nitrofurntoin across a mouse mammary epithelial monolayer.J. Pharmacol. Exp. Ther., 280, 669–676 (1997).
Wilson, J. T., Determinants and consequences of drug excretion in breast milk.Drug Metab. Rev., 14, 619–652 (1983).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimura, S., Morimoto, K., Okamoto, H. et al. Development of a human mammary epithelial cell culture model for evaluation of drug transfer into milk. Arch Pharm Res 29, 424–429 (2006). https://doi.org/10.1007/BF02968594
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02968594