Abstract
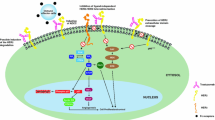
The HER-2/neu protein is thought to be a unique and useful target for antibody therapy of cancers overexpressing the HER-2/neu gene. The recombinant humanized arti-HER-2 monoclonal antibody, trastuzumab (HerceptinTM) is now available for clinical use in the U.S.A. It is also expected to be available in Japan in the near future. In this paper, the details of this novel biologic agent are reviewed in conjunction with a phase I study performed in Japan.
Similar content being viewed by others
References
Yarden Y, Ullrich A: Growth factor receptor tyrosine kinases.Annu Rev Biochem 57:443–478, 1988.
Coussens L, Yang-Feng TL, Iiao Y-C,et al: Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene.Science 230:1132–1139, 1985.
Yamamoto T, Ikawa S, Akiyama T,et al: Similarity of protein encoded by human c-erbB-2 gene to epidermal growth factor receptor.Nature 319:230–234, 1986
King CR, Kraus MH, Aronson SA Amplification of a novel v-erbB-related gene in a human mammary carcinoma.Science 229:974–976, 1985.
Slamon DJ, Godolphin W, Jones LA,et al: Studies of th.HER-2/neu proto-oncogene in human breast and ovarian cancer.Science 244:707–712, 1989.
De Porer CR, Van Daele S, Van De Vijver MJ,et al: The expression of the neu oncogene in breast lesions and in normal fetal and adult human tissues.Histopathology 15:315–326, 1989.
Prss MF, Cerdon-Cardo C, Slamon DJ: Expression of th.HER-2/neu proto-oncogene in normal human adult and fetal tissues.Oncogene 5:953–962, 1990.
Drebin JA, Link VC, Stern DF,et al: Down modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies.Cell 41:695–706, 1985.
Hudziak RM, Lewis GD, Winget M,et al: pl85HER2 monoclonal antibody has antiproliferative effects in vitro and sensitized human breast tumor cells to tumor necrosis factor.Mol Cell Biol 9:1165–1172, 1989.
Hancock MC, Langton BC, Chan T,et al: A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cis-diamminedichloroplatinum against human breast and ovarian tumour cell lines.Cancer Res 51:4575–4580, 1991.
Stancovski I, Hurwitz E, Leitner O,et al: Mechanistic aspects of the opposing effects of monoclonal antibodies to the ERBB-2 receptor on tumor growth.Proc Natl Acad Sci USA 88:8691–8695, 1991.
Tagliabue E, Centis F, Campiglio M,et al: Selection of monoclonal antibodies which induce internalization and phosphorylation of pl85HER2 and growth inhibition of cells wit.HER2/neu gene amplification. Int J Cancer 47:933–937, 1991.
Harwerth I-M, Wels W, Marte BM,et al: Monoclonal antibodies against the extracellular domain of th.erbB-2 receptor function as partial ligand agonists.J Biol Chem 267:15160–15167, 1992.
Kasprzyk PG, Song SU, Di Fiore PP,et al: Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2monoclonal antibodies.Cancer Res 52:2771–2776, 1992.
Schroff RW, Foon KA, Beatty SM,et al: Human antimurine immunoglobulin responses in patients receiving monoclonal antibody therapy.Cancer Res 45:879–885, 1985.
Ohnish Y, Nakamura H, Yoshimura M,et al: Prolonged survival of mice with human gastric cancer treated with an anti-c-erbB-2 monoclonal antibody.Br J Cancer 71:969–973, 1995.
Shawler DL, Bartholomew RM, Smith LM,et al: Human immune response to multiple injection of murine monoclonal IgG.J Immunol 135:1530–1535, 1985.
Carter P, Presta L, Gorman CM,et al: Humanization of an anti-pl85HEB2 antibody for human cancer therapy.Proc Natl Acad Sci USA 89:4285–4289, 1992.
Tokuda Y, Ohnish Y, Shimamura K,et al: In vitro an.in vivo anti-tumour effects of a humanised monoclonal antibody against c-erbB-2 product.Br J Cancer 73:1362–1365, 1996.
Cobleigh MA, Vogel CL, Tripathy D,et al: Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who hav.HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease.J Clin Oncol 17:2639–2648, 1999.
Tokuda Y, Watanabe T, Omuro Y,et al: Dose escalation and pharmacokinetic study of a humanised anti-HER2 monoclonal antibody in patients wit.HER2/neu-over-expressing metastatic breast cancer.Br J Cancer 81:1419–1425, 1999.
Vogel C, Cobleigh M, Tripathy D,et al: First-line, non-hormonal, treatment of women withHER2 over-expressing metastatic breast cancer with Herceptin (trastuzumab, humanized anti-HER2 antibody).Proc Am Soc Clin Oncol 19:71a, 2000 (abstr 275).
Pegram M, Hsu S, Lewis G,et al: Inhibitory effects of combinations o.HER-2/neu antibody and chemother-apeutic agents used for treatment of human breast cancers.Oncogene 18:2241–2251, 1999.
Slamon D, Leyland-Jones B, Shak S,et al: Addition of HerceptinTM (humanized anti-HER2 antibody) to first line chemotherapy forHER2 overexpressing metastatic breast cancer(HER2 + /MBC) markedly increase anticancer activity: a randomized multinational controlled phase III trial.Proc Am Soc Clin Oncol 17:98a, 1998 (abstr 377).
Seidman A, Fornier M, Esteva F,et al: Final report: weekly (W) Herceptin and Taxol (T) for metastatic breast cancer (MBC): analysis of efficacy byHER2 immunophenotype (immunohistochemistry (IHC) and gene amplification (fluorescent in-situ hybridization (FISH)).Proc Am Soc Clin Oncol 19:83a, 2000 (abstr 319).
Burstein H, Kuter I, Richardson P,et al: Herceptin and vinorelbine forHER2-positive metastatic breast cancer: a phase II study.Proc Am Soc Clin Oncol 19:102a, 2000 (abstr 392).
Mass RD, Sanders C, Charlene K,et al: The concordance between the clinical trials assay (CTA) and fluorescence in situ hybridization (FISH) in the Herceptin pivotal trials.Proc Am Soc Clin Oncol 19:75a, 2000 (abstr 291).
Buehler H, Bangemann N, Evers K,et al: EffectiveHER-2/neu diagnosis in breast cancer by a combination of immunohistochemistry and FISH.Proc Am Soc Clin Oncol 19:76a, 2000 (abstr 294).
Author information
Authors and Affiliations
About this article
Cite this article
Tokuda, Y., Ohta, M., Suzuki, Y. et al. Clinical development of trastuzumab in Breast Cancer. Breast Cancer 8, 93–97 (2001). https://doi.org/10.1007/BF02967486
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02967486