Abstract
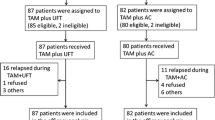
A multi-center, randomized controlled collaborative study was conducted in 310 institutions located throughout Japan for 3 years and 9 months from February 1985 until October 1988 to evaluate the efficacy of post-operative adjuvant therapy for patients who had previously undergone curative surgery for treatment of Stage IIIa breast cancer. Patients with estrogen receptor-positive [ER( + )] breast cancer were treated with two types of regimens, ie, cyclophosphamide + adriamycin + fluorouracil (CAF; 2 cycles) + Futraful (FT) or CAF (2 cycles) + FT + tamoxifen (TAM), and the clinical benefit of additional use of TAM was evaluated. Of the 509 ER( + ) patients registered for the trial, 473 patients (92.9%) were eligible for evaluation. The 5-year survival rate was 77.2% for the CAF + FT group and 74.6% for the CAF + FT+TAM group, and the 5-year disease-free survival rate was 56.7% for the CAF+FT group and 59.2% for the CAF + FT + TAM group. Neither the survival rate nor the disease-free survival rate differed significantly between the groups. Analyses by factor revealed that the 5-year disease-free rate for lymph node-negative patients in the CAF + FT + TAM group was significantly higher than that for the corresponding patients in the CAF + FT group. No differences were noted in the incidence of adverse reactions between the two treatment groups, other than an increase in LDH (the frequency of which was higher in the CAF + FT+TAM group than in the CAF + FT group). Patients with estrogen receptor-negative [ER( -)] breast cancer were treated with two types of regimens, ie, CAF + FT or CAF + FT + adriamycin (ADR), and the clinical benefit of the combined use of intermittent doses of ADR was evaluated. Of the 514 ER(-) patients registered in the trial, 478 (93.0%) were eligible for evaluation. The 5-year survival rate was 64.9% for the CAF + FT group and 63.0% for the CAF + FT + ADR group, and the 5-year disease-free survival rate was 59.2% for both CAF + FT and CAF + FT + ADR groups. Neither the survival rate nor the disease-free survival rate differed significantly between the groups. There were no significant differences between these groups in analyses by nodal or menopausal status. The incidences of adverse reactions including anorexia, nausea/vomiting and alopecia were higher in the CAF + FT+ADR group than in the CAF + FT group.
Similar content being viewed by others
Abbreviations
- ACETBC:
-
Adjuvant Chemoendocrine Therapy for Breast Cancer
- ER:
-
Estrogen receptor
- TAM:
-
Tamoxifen
- FT:
-
Futraful
- ADR:
-
Adriamycin
- CAF:
-
Cyclophosphamide + adriamycin + fluorouracil
- CMF:
-
Cyclophosphamide + methotrexate + fluorouracil
- MMC:
-
Mitomycin-C
- EBCTCG:
-
Early Breast Cancer Trialists’ Collaborative Group
References
Goldhirsch A, Wood WC, Senn H,et al: Meeting highlights; International consensus panel on the treatment of primary breast cancer.JNCI 87(19):1441–1445, 1995.
Abe O: The role of chemoendocrine agents in postoperative adjuvant therapy for breast cancer; Meta-analysis of the first collaborative studies of postoperative adjuvant chemoendocrine therapy for breast cancer (ACETBC).Breast Cancer l(l):1–9, 1994.
Yokoe T: Current Diagnosis and Treatment of Breast Cancer, Izuo Med, Nagai-Syoten, Tokyo, pp185–195, 1990.
Yamamoto H: Controversies in the surgical, options for the patient with primary breast cancer.Jpn J Cancer Chemother 21(16):2720–2727, 1994.
Carter SK: Chemotherapy of breast cancer; Current status. In: Meuson JC ed, Breast Cancer Trends in Research and Treatment, Raven Press, New York, pp193–215, 1976.
Bull JM, Tormey DC, Shou-Hua L,et al: A randomized comparative trial of adriamycin versus methotrexate in combination drug therapy.Cancer 41(5): 1649–1657, 1978.
Early Breast Cancer Trialists’ Collaborative Group: Treatment of Early Breast Cancer, vol. 1; Worldwide Evidences 1985-1990, Oxford University Press, Oxford, pp12–18, 1990.
Abe O, Izuo M, Enomoto K,et al: Combination chemotherapy of cyclophosphamide adriamycin and 5-fluorouracil (CAF) in advanced and recurrent breast cancer.Jpn J Cancer Chemother 9(5):866–873, 1982.
Toi M, Hattori T, Akagi M,et al: Randomized adjuvant trial to evaluate the addition of tamoxifen and PSK to chemotherapy in patients with primary breast cancer.Cancer 70(10): 2475–2483, 1992.
Boccardo F, Rubagotti A, Bruzzi P,et al: Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node-positive, estrogen receptor-positive breast cancer patients; Results of a multicentric Italian study.J Clin Oncol 8(8):1310–1320, 1990.
Abe R, Tsuchiya A, Koie H,et al: A cooperative randomized controlled study of adjuvant chemoendocrine therapy for breast cancer in Japan.Am J Clin Oncol 17(2):103–108, 1994.
Hubay CA, Gordon NH, Crowe JP,et al: Antiestrogen-cytotoxic chemotherapy and bacillus Calmette-Guerin vaccination in stage II breast cancer; Seventy-two month follow-up.Surgery 96(l):61–72, 1984.
Fisher B, Redmond C, Brown A,et al: Adjuvant chemotherapy with and without tamoxifen in the treatment of primary breast cancer; 5-Year results from the National Surgical Adjuvant Breast and Bowel Project Trial.J Clin Oncol 4(4): 459–471, 1986.
Kaufmann M, Jonat W, Caffier H,et al: Adjuvant systemic risk adapted cytotoxic± tamoxifen therapy in women with node positive breast cancer. In: Adjuvant Therapy of Cancer V, Grune & Stratton Inc, Orland, pp337–346, 1987.
Early Breast Cancer Trialists’ Collaborative Group: Systemic treatment of early breast cancer by hormonal, cytotoxic or immune therapy.Lancet 339:1–15, 1992.
Jordan C: Antiestrogen therapy for breast cancer. In: Osboren K ed, Endocrine Therapies in Breast and Prostate Cancer, Kluwer Academic Publisher, Boston, pp97–110, 1988.
Fisher B, Redmond C, Wickerham DL,et al: Doxor- ubicin-containing regimens for the treatment of stage II breast cancer; The National Surgical Adjuvant Breast and Bowel Project experience.J Clin Oncol 7(5):572–592, 1989.
Moliterni A, Bonadonna G, Valagussa P,et al: Cyclophosphamide, methotrexate and fluorouracil with and without doxorubicin in the adjuvant treatment resectable breast cancer with one to three positive axillary nodes.J Clin Oncol 19(7):1124–1130, 1991.
Kubo K, Abe O, Izuo M,et al: Clinical evaluation of adriamycin for advanced breast cancer (No. 3); A joint study by 26 institutes on the CAF and CMcF regimens.Jpn J Cancer Chemother 10(12):2523–2531, 1983.
Author information
Authors and Affiliations
About this article
Cite this article
Yoshida, M., Abe, O., Uchino, J. et al. Efficacy of postoperative adjuvant therapy for stage ilia breast cancer: Futraful vs futraful+tamoxifen for er-positive patients and futraful vs futraful + adriamycin for er-negative breast cancer. Breast Cancer 4, 103–113 (1997). https://doi.org/10.1007/BF02967063
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02967063