Abstract
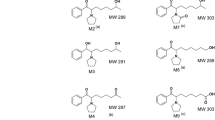
An alarming increase in the misuse/abuse of nitrobenzodiazepine derivatives, especially flunitrazepam, prompted us to establish reliable analytical protocols for their routine detection. Whilst the parent drugs are readily available from a number of commercial sources, it was found difficult to obtain samples of the corresponding amino metabolites which were required as analytical standards. This lead us to develop the straightforward synthetic protocol described here, to convert the readily available parent drugs, namely flunitrazepam and nitrazepam, to their respective 7-amino derivatives. The method requires minimum laboratory facilities. It involves the reduction of the nitro functionality in the parent drug to an amino group using tin (II) chloride under mild conditions, using ultrasonication at room temperature. The method is simple and should give toxicology laboratories better access to these much needed compounds.
Similar content being viewed by others
References
Martindale, The Extra Pharmacopoeia, 31st edition. Royal Pharmaceutical Society, 1999; 669–744.
Anglin, D., Spears, K. L., Range Hutson, H. Flunitrazepam and its involvement in date or acquaintance rape. Acad. Emerg. Med. 1997; 4: 323–326.
ElSohly, M. A., Feng, S., Salamone, S. J., Wu, R. A sensitive GC-MS method for the analysis of flunitrazepam and its metabolites in urine. J. Anal. Tox. 1997; 21: 335–340.
Doxsee, K. M., Feigel, M., Stewart, K. D., Canary, J. W., Knobler, C. B., Cram, D. J. Host-guest complexation. 42. Preorganization strongly enhances the tendency of hemispherands to form hemispheraplexes. J. Am. Chem. Soc. 1987; 109: 3098–3107.
Bellamy, F. D., Ou, K. Selective reduction of aromatic nitro compounds with stannous chloride in non acidic and non aqueous medium. Tet. Lett. 1984; 25: 839–842.
Scholl, H., Kloster, G., Stocklin, G. Bromine-75 labelled benzodiazepines: Potential agents for mapping of benzodiazepine receptorsin vivo. J. Nucl. Med. 1983; 24: 417–422.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Feely, J., Kavanagh, P.V., McNamara, S.M. et al. Simple preparation of the major urinary metabolites of flunitrazepam and nitrazepam. Ir. J. Med. Sc. 168, 8–9 (1999). https://doi.org/10.1007/BF02939571
Issue Date:
DOI: https://doi.org/10.1007/BF02939571