Abstract
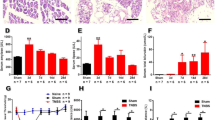
The density and distribution of N-methyl-D-aspartate receptor (NMDAR1) mRNA expression in the rat midbrain Periaqueductal gray (PAG) following exposure to unilateral peripheral inflammation or chronic constrictive injury (CCI) as models for chronic peripheral nociception were examined using thein situ hybridization technique. The NMDAR1 hybridization signal intensities increased significantly in the ventrolateral areas of the caudal and middle thirds of the PAG after 3 days of Complete Freund’s Adjuvant (CFA) injection. Likewise, rats subjected to CCI showed significant increases in hybridization signal intensities in comparison to sham operated animals in both the ipsi and contra-lateral ventrolateral quadrants of the caudal and middle thirds of the PAG. In the caudal dorsal raphe, the CFA and the CCI treated animals showed a significant increase in signal hybridization compared to control and sham operated groups while the rostral dorsal raphe showed no significant changes in either CCI or CFA treated groups. In contrast, there was no significant change in signal intensity of NMDAR1 mRNA in the dorsal subdivisions of the PAG following either CCI or CFA treatment. These results demonstrate significant bilateral increase in NMDAR1 mRNA expression in the ventrolateral areas of the caudal and middle thirds of the PAG and the caudal half of the dorsal raphe following chronic nociception. The up-regulation phenomenon may constitute a reactive mechanism against chronic neuropathic pain in the PAG.
Similar content being viewed by others
References
Betz, H. Ligand-gated ion channels in the brain: the amino acid receptor superfamily. Neuron. 1990; 5: 383–392.
Jacquet, Y. F. The NMDA receptor: Central role in pain inhibition in the rat periaqueductal gray. Eur. J. Pharmacol 1988; 154: 271–276.
Behbehani, M. M., Fields, H. L. Evidence that an excitatory connection between periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979; 170: 85–93.
Beitz, A. J. Relationship of glutamate and aspartate to the periaqueductal gray raphe magnus projection: analysis using immunocytochemistry and microdialysis. J. Histochem. Cytochem. 1990; 38: 1755–1765.
Jensen, T. S., Yaksh, T. L. The antinociceptive activity of excitatory amino acids in the rat brainstem: an anatomical and pharmacological analysis. Brain Res. 1992; 569: 255–267.
Wiklund, L., Behzadi, G., Kalen, P., Headley, P. M., Nicolopoulos, L. S., Parsons, C. G., West, D. C. Autoradiographic and electrophysiological evidence for excitatory amino acid transmission in the periaqueductal gray projection to nucleus raphe magnus in the rat. Neurosci. Lett. 1988; 93: 158–163.
Aanoson, L., Wilcox, G. L. Nociceptive action of excitatory amino acids in the mouse: effects of spinally administered opioids, phencyclidine and sigma agonists. J. Pharmacol. Exp. Ther. 1987; 243: 9–19.
Eisenberg, E., LaCross, S., Strassman, A. M. The effects of the clinically tested NMDA receptor antagonist memantine on carrageenan-induced thermal hyperalgesia in rats. Eur. J. Pharmacol. 1994; 255: 123–129.
Neugebauer, V., Lucke, T., Grubb, B., Schaible, H. G. The involvement of N-methyl-D-aspartate (NMDA) and non-NMDA receptors in the responsiveness of rat spinal neurons with input from the chronically inflamed ankle. Neurosci. 1994; 170: 237–240.
Ren, K., Dubner, R. NMDA receptor antagonists attenuate mechanical hyperalgesia in rats with unilateral inflammation of the hindpaw. Neurosci. Lett. 1993; 163: 22–26.
Bennett, G. J. Evidence from animal models on the pathogenesis of painful peripheral neuropathy: relevance for pharmacotherapy. In: A. I. Basbaum and J. M. Besson (Eds ), Towards a New Pharmacotherapy of Pain, John Wiley 1991; 365–379.
Dubner, R. Neuronal plasticity and pain following peripheral tissue inflammation or nerve injury. In: M. R. Bond, J. E. Charlton and C. J. Woolf (Eds.) Proc. of the VIth World Congress on Pain, Amsterdam, Elseveir. 1991; 263–276.
Seltzer, Z., Cohn, S., Ginszberg, R., Beilin, B. Z. Modulation of neuropathic pain behavior in rats by spinal disinhibition and NMDA receptor blockade of injury discharge. Pain 1991; 45: 69–75.
Tal, M., Bennett, G. J. Dextrophan relieves neuropathic heat-evoked hyperalgesia in the rat. Neurosci. Lett. 1993; 151: 107–110.
Yamamoto, T., Yaksh, T. L. Comparison of the antinociceptive effects of preand post-treatment with intrathecal morphine and MK801, and NMDA antagonist, on the formalin test in the rat. Anesthesiol. 1992; 77: 757–763.
Sluka, K. A., Jordan, H. H., Willis, W. D., Westlund, K. N. Differential effects of N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists on spinal release of amino acids after development of acute arthritis in rats. Brain Res. 1994; 664: 77–84.
Ishii, T., Moriyoshi, K., Sugihara, H., Sakurada, K., Kadotani, H., Yokoi, M., Akazawa, C., Shigemoto, R., Mizuno, N., Masu, M., Nakanishi, S. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J. Biol. Chem.1993; 268: 2836–2843.
Moriyoshi, K., Masu, M., Ishii, T., Shigemoto, R., Misuno, Nakanishi, S. Molecular cloning and characterization of the rat NMDA receptor. Nature 1991 ; 354: 31–37.
Beitz, A. J. The midbrain periaqueductal gray in the rat. I. Nuclear volume, cell density, orientation, and regional subdivisions. J. Comp. Neur. 1985; 237: 445–459.
Hamilton, B. L. Cytoarchitectural subdivisions of the periaqueductal gray matter in the cat. J. Comp. Neuro. 1973; 149: 1–28.
Shiply, M. T., McLean, J. H., Behbehani, M. M. Heterogeneous distribution of neurotensin-like immunoreactive neurons and fibers in the midbrain periaqueductal gray of the rat. J. Neurosci. 1987; 7: 2025–2034.
Fardin, V., Oliveras, J. L., Besson, J. M. A. Reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain Res. 1984, 306: 105–123.
Mayer, D. J., Wolfle, T. L., Akil, H., Garder, B., Liebeskind, J. C. Analgesia from electrical stimulation in the brainstem of the rat. Sci. 1971; 174: 1351–1354.
Depaulis, A., Morgan, M. M., Liebeskind, G. A. B. Aergic modulation of the analgesic effects of morphine microinjected in the ventral periaqueductal gray matter of the rat. Brain Res. 1987; 436: 223–228.
Carrive, P., Dampney, R. A. L., Bandler, R. Excitation of neurons in a restricted portion of the midbrain periaqueductal gray elicits both behavioral and cardiovascular components of the defense reaction in the unanaesthetised decerebrate cat. Neurosci. Lett. 1987; 81: 273–278.
Larson, C. R., Kistler, M. K. The relationship of periaqueductal gray neurons to vocalization and laryngeal EMG in the behaving monkey. Exp. Brain Res. 1986; 63: 596–606.
Basbaum, A. I., Fields, H. L. Endogenous pain control system: Brainstem spinal pathways and endorphin circuitry. Ann. Rev. Neurosci. 1984; 7: 309–338.
Keay, K. A., Bandler, R. Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal gray of the rat. Neurosci. Lett. 1993; 154: 23–26.
Keay, K. A., Clement, C. I., Owler, B., Depaulis, A., Bandler, R. Convergence of deep somatic and visceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neurosci. 1994; 61: 727–732.
Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110.
Williams, F., Beitz, A. J. Chronic pain increases brainstem proneurotensin/neruomedin-N mRNA expression: a hybridization-histochemical and immunohistochemical study using three different rat models for chronic nociception. Brain Res. 1993; 611:87–102.
Stein, C., Millan, J. J., Herz, A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: Alterations in behavior and nociceptive thresholds. Pharmacol. Biochem. Behav. 1988; 31: 445–451.
Bennett, G. J., Xie, Y. K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87–107.
Noguchi, K., Ruda, M. A. Gene regulation in an ascending nociceptive pathway: inflammation-induced increase in preprotachykinin mRNA in rat lamina I spinal projection neurons. J. Neurosci. 1992; 7: 2563–2572.
Reichling, D. B., Basbaum, A. I. Collateralization of periaqueductal gray neurons to forebrain or diencephalon and to the medullary nucleus raphe magnus in the rat. Neurosci. 1991; 42: 183–200.
Paxinos, G, Watson, C. The rat brain stereotaxic coordinates. (2nd Edn) Sydney; Academic Press. 1986.
Harrison, P. J., Pearson, R. C. In situ hybridization histochemistry and the study of gene expression in the human brain. Prog. Neurobiol. 1990; 34: 271–312.
Palacios, J. M., Mengod, G., Vilaro, M. T., Ramm, R. Recent trends in receptor analysis techniques and instrumentation. J. Chem. Neuroanat. 1991; 4: 343–353.
Renno, W. M., Kus, L., Price, R., Beitz, A. J. Analysis of NMDA receptor mRNA expression in the caudal periaqueductal gray of rats in two pain models. In IASP Publications, Congress Abstracts 7th world Congress on Pain, Paris, France; 1993.
Sherman, A. D., Gebhart, G. F. Pain-induced alteration of glutamate in periaqueductal central gray and its reversal by morphine. Life Sci. 1975; 15: 1781–1789.
Cotman, C. W., Monaghan, D. T., Ottersen, O. P., Storm-Mathisen, J. Anatomical organization of excitatory amino acid receptors and their pathways. Trends Neurosci. 1987; 10: 273–280.
Clements, J. R., Madl, J. E., Johnson, R. L., Larson, A. A, Beitz, A. J. Localization of glutamate, glutaminase, aspartate and aspartate aminotransferase in the rat midbrain periaqueductal gray. Exp. Brain Res. 1987; 67: 594–602.
Beitz, A. J., Williams, F. G. Localization of putative amino acid transmitters in the PAG and their relationship to the PAG-raphe magnus pathway. In: A. Depaulis and R. Bandler (Eds.), The Midbrain Periaqueductal Gray Matter, New York, Plenum, 1991; 305–327.
Renno, W. M., Mullett, M. A., Beitz, A. J. Systemic morphine reduces GABA release in the lateral but not the medial portion of the midbrain periaqueductal gray of the rat. Brian Res. 1992; 594: 221–232.
Ottersen, O. P., Storm-Mathisen, J. Glutamate-and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J. Comp. Neurol. 1984; 229: 374–392.
Beart, P. M., Summers, R. J., Stephenson, J. A., Cook, C. J., Christie, M. J. Excitatory amino acid projections to the periaqueductal gray in the rat; a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neurosci. 1990; 34:63–176.
Christie, M. J., James, L. B, Beart, P. M. An excitatory amino acid projection from rat prefrontal cortex to periaqueductal gray. Brain Res. Bull. 1986; 16: 127–129.
Beart, P. M., Nicolopoulos, L. S., West, D. C, Headley, P. M. An excitatory amino acid projection from the ventromedial hypothalamus to periaqueductal gray in the rat: autoradiographic and electrophysiological evidence. Neurosci. Lett. 1988; 85: 205–211.
Beitz, A. J. Possible origin of glutamatergic projection to the midbrain periaqueductal gray and deep layer of the superior colliculus of the rat. Brain Res. Bull. 1989; 23: 25–35.
Hardy, S. G. P. Analgesia elicited by prefrontal stimulation. Brain Res. 1985; 339: 281–284.
Al-Rodhan, N., Chipkin, R., Yaksh, T. L. The antinociceptive effects of SCH-32615, a neutral endopeptidase (enkephalinase) inhibitor, microinjected into the periaqueductal, ventral medulla and amygdala. Brain Res. 1990; 520: 123.
Aimone, L. D., Gebhart, G. F. Serotonin and/or an excitatory amino acid in the medial medulla mediates stimulation-produced antinociception from the lateral hypothalamus in the rat. Brain Res. 1988; 450: 170.
Mayer, D. J., Price, D. D. Central nervous system mechanism of analgesia. Pain 1976; 2: 379–404.
Fang, F. G., Haws C. M., Drasner, K., Williamson, A, Fields, H. L. Opioid peptides (DAGO-enkephalin, dynorphin A( 1 –13), BAM 22P) microinjected into the rat brainstem: comparison of their antinociceptive effect and their on neuronal firing in the rostral ventrolateral medulla. Brain Res. 1989; 501: 116.
Andersen, E., Dafny, N. An ascending serotoninergic pain modulation pathway from the dorsal raphe nucleus to the parafascicularis nucleus of the thalamus. Brain Res. 1983; 269: 57–67.
Oliveras, J. L., Besson, J. M. Stimulation-produced analgesia in animals: behavioral investigations: In H. L. Feilds and J. M. Besson (Eds.), Pain Modulation, Progress in Brain Research Vol. 77, Amsterdam, Elseveir. 1988; 141–157.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renno, W.M. Prolonged noxious stimulation increases periaqueductal gray NMDA MRNA expression: A hybridization study using two different rat models for nociception. Ir. J. Med. Sc. 167, 181–192 (1998). https://doi.org/10.1007/BF02937933
Issue Date:
DOI: https://doi.org/10.1007/BF02937933