Abstract
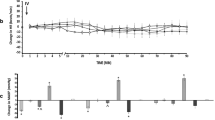
Endothelin-1 has been reported to be responsible for gastric mucosal damage in various experimental models. We evaluated the role of endogenous endothelin-1 in the pathogenesis of gastric mucosal damage induced by indomethacin and HCl in the rat. Rats were given indomethacin (25 mg/kg) subcutaneously, and 15 min later, 0.2N HCl intragastrically. Gastric mucosal damage, gastric endogenous endothelin-1, and gastric mucosal hemodynamics were measured. The effects of bosentan, a mixed endothelin receptor antagonist, on gastric mucosal integrity and hemodynamics were assessed. Gastric endogenous endothelin-1 was significantly elevated at 20min, gastric mucosal blood flow began to decrease significantly at 25 min, and gastric damage occupied 52.2% of the total glandular mucosa at 135 min after injection of indomethacin. Intragastric pretreatment with bosenthan (5, 10, 30, and 60 mg/kg) significantly attenuated gastric damage, to 26.1%, 7.7%, 3.6%, and 1.6%, respectively, of the total glandular mucosa. Bosentan (60 mg/kg) prevented the initial decrease of blood flow and, even at 135 min, improved blood flow and hemoglobin oxygen saturation significantly. We suggest that indomethacin-induced endogenous endothelin-1 diminishes gastric mucosal blood flow and tissue oxygenation and ultimately causes gastric damage. Endogenous endothelin-1 may play an important role in the pathogenesis of the acute gastric mucosal lesions induced by indomethacin and HCl.
Similar content being viewed by others
References
Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411–415.
Wallace JL, Cirino G, de Nucci G, et al. Endothelin has potent ulcerogenic and vasoconstrictor actions in the stomach. Am J Physiol 1989;256:G661-G666.
Morales RE, Johnson BR, Szabo S. Endothelin induces vascular and mucosal lesions, enhances the injury by HCl/ethanol, and the antibody exerts gastroprotection. FASEB J 1992;6:2354–2360.
Masuda E, Kawano S, Nagano K, et al. Role of endogenous endothelin in pathogenesis of ethanol-induced gastric mucosal injury in rats. Am J Physiol 1993;265:G474-G481.
Michida T, Kawano S, Masuda E, et al. Role of endothelin in 1 hemorrhagic shock-induced gastric mucosal injury in rats. Gastroenterology 1994;106:988–993.
Kitajima T, Tani K, Yamaguchi T, et al. Role of endogenous endothelin in gastric mucosal injury induced by hemorrhagic shock in rats. Digestion 1995;56:111–116.
Kitajima T, Yamaguchi T, Tani K, et al. Role of endothelin and platelet-activating factor inindomethacin-induced gastric mucosal injury in rats. Digestion 1993;54:156–159.
Clozel M, Breu V, Burri K, et al. Pathophysiological role of endothelin revealed by the first orally active endothelin receptor antagonist. Nature 1993;365:759–761.
Clozel M, Breu V, Gray GA, et al. Pharmacological characterization of bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther 1994;270:228–235.
Lazaratos S, Nakahara A, Goto K, et al. Bosentan antagonizes the effects of endothelin-1 on rat gastric blood flow and mucosal integrity. Life Sci 1995;56:PL195-PL200.
Kitamura K, Tanaka T, Kato J, et al. Immunoreactive endothelin in rat kidney inner medulla: Marked decrease in spontaneously hypertensive rats. Biochem Biophys Res Commun 1989;162:38–44.
Iwata R, Ito H, Hayashi T, et al. Stable and general-purpose chemiluminescent detection system for horseradish peroxidase employing a thiazole compound enhancer and some additives. Anal Biochem 1995;231:170–174.
Iwata R, Hayashi T, Higuchi A, et al. Sensitive chemiluminescent enzyme immunoassay for determination of endothelin-1. Jpn J Clin Chem 1996;25:91–96.
Lazaratos S, Kashimura H, Nakahara A, et al. Gastric ulcer induced by submucosal injection of ET-1: role of potent vasoconstriction and intraluminal acid. Am J Physiol 1993;265:G491-G498.
Takeuchi K, Niida H, Ohuchi T, et al. Influences of urethane anesthesia on indomethacin-induced gastric mucosal lesions in rats: Relation to blood glucose levels. Dig Dis Sci 1994;39:2536–2542.
Robert A, Nezamis JE, Lancaster C, et al. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 1979;77:433–443.
Chadhury TK, Robert A. Prevention by mild irritants of gastric necrosis produced in rats by sodium taurocholate. Dig Dis Sci 1980;25:830–836.
Matsumoto J, Takeuchi K, Ueshima K, et al. Role of capsaicin-sensitive afferent neurons in mucosal blood flow response of rat stomach induced by mild irritants. Dig Dis Sci 1992;37:1336–1344.
Ihara M, Noguchi K, Saeki T, et al. Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci 1992;50:247–255.
Filep JG, Clozel M, Fournier A, et al. Characterization of receptors mediating vascular responses to endothelin-1 in the conscious rat. Br J Pharmacol 1994;113:845–852.
Takeuchi K, Ueshima K, Hironaka Y, et al. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Digestion 1991;49:175–184.
Kauffman GL, Aures D, Grossman MI. Intravenous indomethacin and aspirin reduce basal gastric mucosal blood flow in dogs. Am J Physiol 1980;238:G131-G134.
Arakawa T, Nakamura H, Chono S, et al. Absence of effect of 16,16-dimethyl prostaglandin E2 on reduction of gastric mucosal blood flow caused by indomethacin in rats. Dig Dis Sci 1989; 34:1369–1373.
Lazaratos S, Hassan M, Matsumaru K, et al. The role of endogenous ET-1 in the indomethacin-induced reduction in gastric mucosal blood flow. Gut 1995;37[Suppl 2]:A149.
Richard V, Hogie M, Clozel M, et al. In vivo evidence of an endothelin-induced vasopressor tone after inhibition of nitric oxide synthesis in rats. Circulation 1995;91:771–775.
Teerlink JR, Carteaux JP, Sprecher U, et al. Role of endogenous endothelin in normal hemodynamic status of anesthetized dogs. Am J Physiol 1995;268:H432-H440.
Lawrence E, Brain SD. Effect of BQ-123 and Ro 47-0203 (bosentan) on endothelin-induced vasoconstriction in the rat skin. Eur J Pharmacol 1994;260:103–106.
Takayanagi R, Kitazumi K, Takasaki C, et al. Presence of non-selective type of endothelin receptor on vascular endothelium and its linkage to vasodilation. FEBS Lett 1991;282:103–106.
Clozel M, Gray GA, Breu V, et al. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem Biophys Res Commun 1992;186:867–873.
Adachi M, Furuichi Y, Miyamoto C. Identification of a region of the human endothelin ETA receptor required for interaction with bosentan. Eur J Pharmacol 1994;269:225–234.
Lopez-Belmonte J, Whittle BJR. The involvement of endothelial dysfunction, nitric oxide and prostanoids in the rat gastric microcirculatory responses to endothelin-1. Br J Pharmacol 1994; 112:267–271.
Grega GJ, Adamski SW, Dobbins DE. Physiological and pharmacological evidence for the regulation of permeability. Fed Proc 1986;45:96–100.
Takeuchi K, Ueki S, Okabe S. Importance of gastric motility in the pathogenesis of indomethacin-induced gastric lesions in rats. Dig Dis Sci 1986;31:1114–1121.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsumaru, K., Kashimura, H., Hassan, M. et al. Bosentan, a novel synthetic mixed-type endothelin receptor antagonist, attenuates acute gastric mucosal lesions induced by indomethacin and HCl in the rat: Role of endogenous endothelin-1. J Gastroenterol 32, 164–170 (1997). https://doi.org/10.1007/BF02936362
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02936362