Abstract
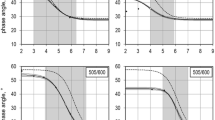
The pH of microbial culture medium was calculated from equations of equilibrium, material balances for ionic components and electro-neutrality theory. Ammonium ion consumption and Acetic acid production are found out to be the major contributors the alteration of the pH as well as the buffer capacity of the medium. By measuring the buffer capacity on-line, levels of acetic acid were estimated by a software sensor using pH signal in a fermentation process of E.coli growing in a minimal medium. The measured values of acetic acid showed good correlation to those of estimated by the software sensor.
Similar content being viewed by others
Abbreviations
- [H2PO4 −] consume :
-
The quantity of dibasic phosphate ion utilized for microbial growth (M)
- [H2PO4 −] i :
-
Initial concentration of monobasic phosphate ion provided (M)
- [HA] p :
-
Organic acid produced (M)
- Hco2 :
-
Henry’s law constant of carbon dioxide (M/atm)
- [HPO4 −] consume :
-
The quantity of monobasic phosphate ion utilized for microbial growth (M)
- k 1 :
-
First acidity constant of phosphoric acid
- k 2 :
-
Second acidity constant of phosphoric acid
- k 3 :
-
Third acidity constant of phosphoric acid
- ka AcOH :
-
Acidity constant of acetic acid
- k b :
-
Basicity constant of ammonia
- ka NH3 :
-
Acidity constant of ammonia
- kc 1 :
-
First acidity constant of carbonic acid
- kc 2 :
-
Second acidity constant of carbonic acid
- k w :
-
Autoprotolysis constant of water
- [NH3] consume :
-
The quantity of Ammonia utilized for microbial growth (M)
- [NH4 +] i :
-
Initial concentration of ammonium ion provided (M)
- [OH−] adj :
-
The concentration of base added for the adjustment of pH (M)
- Pco2 :
-
Partial pressure of carbon dioxide
- [CH3COOH]:
-
Acetic acid produced
- [NH4 +] supp :
-
Ammonia fed to supplement acid production
- β:
-
Buffer capacity
- [OH] fed :
-
Alkali fed to control pH
- N alk :
-
Normality of alkali
- F pump :
-
Feed rate of alkali pump
- T pump :
-
Duration of pump operation
- V broth :
-
Volume of broth
References
Schügerl, K. (1991) Common instruments for process analysis and control. p. 6–25, In: H-J. Rehm, G. Reed, A. Puhler and P. Stadler (ed.) Diotechnology. Vol. 4. VCH Publishers Inc. New York, NY.
Bradley, J., P. A. Anderson, A. M. Dear, R. E. Ashby and A. P. F. Turner (1989) Glucose biosensors for the study and control of bakers compressed yeast production. p. 47–51. In: N. M. Fish, R. I. Fox and N. F. Thornhill (ed.) Computer applications in Fermentation Technology. Elsevier Science Publicshers LTD. New York, NY.
Ozturk, S. S., J. C. Thrift, J. D. Blackie and D. Naveh (1997) Real-time monitoring and control of glucose and lactate concentrations in a mammalian cell perfusion reactor.Biotechnol. and Bioeng. 53: 371–378.
Lasko, D. R. and D. I. C. Wang (1996) On-line monitoring of intracellular atp concentration in escherichia coli fermentations.Biotechnol. and Bioeng. 52: 364–372.
Chattaway, T. and G. N. Stephanopoulos (1988) An adaptive state estimator detecting contaminants in bioreactor.Biotechnol. Bioleng. 34: 647–659.
Flaus, J. M. and A. Cheruy (1989) Estimation of the state and parameters of a bioprocess using the Recursive Prediction Error method.proc IFAC Symp.Adv Inf. Proc. Autom. Ctr. 2: 402–407.
Won Hur (1997) Analysis of pH change of phosphate buffered medium caused by cell growth.Kor. J. Biotech. Bioeng. 12: 167–175.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, Y.K., Hur, W. Analysis of pH change and an automatic pH control with A new function: On-line estimation of acetic acid. Biotechnol. Bioprocess Eng. 2, 69–72 (1997). https://doi.org/10.1007/BF02932326
Issue Date:
DOI: https://doi.org/10.1007/BF02932326