Abstract
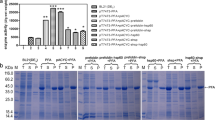
When the alginate lyase gene (aly) fromPseudoalteromonas elyakovii was expressed inE. coli, most of the gene product was organized as aggregated insoluble particles known as inclusion bodies. To examine the effects of chaperones on soluble and nonaggregated form of alginate lyase inE. coli, we constructed plasmids designed to permit the coexpression ofaly and the DnaK/DnaJ/GrpE or GroEL/ES chaperones. The results indicate that coexpression ofaly with the Dnak/DnaJ/GrpE chaperone together had a marked effect on the yield alginate lyase as a soluble and active form of the enzyme. It is speculated this result occurs through facilitation of the correct folding of the protein. The optimal concentration ofl-arabinose required for the induction of the DnaK/DnaJ/GrpE chaperone was found to be 0.05 mg/mL. An analysis of the protein bands on SDS-PAGE gel indicated that at least 37% of total alginate lyase was produced in the soluble fraction when the DnaK/DnaJ/GrpE chaperone was coexpressed.
Similar content being viewed by others
References
Sawabe, T., H. Takahashi, Y. Ezura, and P. Gacesa (2001) Cloning sequence, analysis and expression ofPseudoalteromonas elyakovii IAM 14594 gene (alyPEEC) encoding the extracellular alginate lyase.Carbohydr. Res. 335: 11–21.
Preiss, J., and G. Ashwell (1962) Alginic acid metabolism in bacteria.J. Biol. Chem. 237: 309–316.
Yoon, H. J., W. Hashimoto, O. Miyake, M. Okamoto, B. Mikami, and K. Murata (2000) Overexpression inEscherichia coli, purification, and characterization ofSphingomonas sp. A1 alginate lyases.Protein Expr. Purif. 19: 84–90.
Onsoyen, E. (1996) Commercial applications of alginates.Carbohydr. Eur. 14: 26–31.
Renn, D. (1997) Biotechnology and the red seaweed polysaccharide industry: status, needs and prospects.Trends Biotechnol. 15: 9–14.
Murata, K., T. Inose, T. Hisano, S. Abe, Y. Yonemoto, T. Yamashita, M. Takagi, K. Sakaguchi, A. Kimura, and T. Imanaka (1993) Bacterial alginate lyase: enzymology, genetics and application.J. Ferment. Bioeng. 76: 427–437.
Kim, J. E., E. J. Kim, W. J. Rhee, and T. H. Park (2005) Enhanced production of recombinant protein inEscherichia coli using silkworm hemolymph.Biotechnol. Bioprocess Eng. 10: 353–356.
Jin, H. H., N. S. Han, D. K.Kweon, Y. C. Park, and J. H. Seo (2001) Effects of environmental factors onin vivo folding ofBacillus macerans cyclodextrin glycosyltransferase in recombinantEscherichia coli.J. Microbiol. Biotechnol. 11: 92–96.
Kim, C. I., M. D. Kim, Y. C. Park, N. S. Han, and J. H. Seo (2000) Refolding ofBacillus macerans cyclodextrin glucanotransferase expressed as inclusion bodies in recombinantEscherichia coli.J. Microbiol. Biotechnol. 10: 632–637.
Kondo, A., J. Kohda, Y. Endo, T. Shiromizu, Y. Kurokawa, K. Nishihara, H. Yanagi, T. Yura, and H. Fukuda (2000) Improvement of productivity of active horseradish peroxidase inEscherichia coli by coexpression of Dsb proteins.J. Biosci. Bioeng. 90: 600–606.
Thomas, J. G., A. Ayling, and F. Baneyx (1997) Molecular chaperones, folding catalysis, and the recovery of active recombinant proteins fromE. coli.Appl. Biochem. Biotechnol. 66: 197–238.
Kim, H., and J. H. Kim (2005) Refolding of fusion ferritin by gel filtration chromatography (GFĆ).Biotechnol. Bioprocess Eng. 10: 500–504.
Guan, Y.-X., H.-X. Pan, Y.-G. Gao, S.-J. Yao, and M.-G. Cho (2005) Refolding and purification of recombinant human interferon-γ expressed as inclusion bodies inEscherichia coli using size exclusion chromatography.Biotechnol. Bioprocess Eng. 10: 122–127.
Kwon, M. J., S. L. Park, S. K. Kim, and S. W. Nam (2002) Overproduction ofBacillus macerans cyclodextrin glucanotransferase inE. coli by coexpression of GroEL/ES chaperone.J. Microbiol. Biotechnol. 12: 1002–1005.
Lamark, T., M. Ingebrigtsen, C. Bjornstad, T. Melkko, T. E. Mollnes, and E. W. Nielsen (2001) Expression of active human C1 inhibitor serpin domain inEscherichia coli.Protein Expr. Purif. 22: 349–358.
Park, S. L., M. J. Kwon, S. K. Kim, and S. W. Nam (2004) GroEL/ES chaperone and low culture temperature synergistically enhanced the soluble expression of CGTase inE. coli.J. Microbiol. Biotechnol. 14: 216–219.
Sareen, D., R. Sharma, and R. M. Vohra (2001) Chaper-one-assisted overexpression of an active D-carbamoylase fromAgrobacterium tumefaciens AM 10.Protein Expr. Purif. 23: 374–379.
Hoshino, K., A. Eda, Y. Kurokawa, and N. Shimizu (2002) Production of brain-derived neurotrophic factor inEscherichia coli by coexpression of Dsb proteins.Biosci. Biotechnol. Biochem. 66: 344–350.
Kurokawa, Y., H. Yanagi, and T. Yura (2000) Overex-pression of protein disulfide isomerase DsbD stabilize multiple-disulfide-bonded recombinant protein produced and transported to the periplasm inEscherichia coli.Appl. Environ. Microbiol. 66: 3960–3965.
Han, M. J., S. J. Park, T. J. Park, and S. Y. Lee (2004) Roles and applications of small heat shock proteins in the production of recombinant proteins inEscherichia coli.Biotechnol. Bioeng. 88: 426–436.
Chung, K. T., T. H. Lee, and G. S. Kang (2003) Isolation of proteins that specifically interact with the ATPase domain of mammalian ER chaperone, BiP.Biotechnol. Bioprocess Eng. 8: 192–198.
Dumitru, G. L., Y. Groemping, D. Klostermeier, T. Restle, E. Deuerling, and J. Reinstein (2004) DafA cycles between the DnaK chaperone system and translationalmachinery.J. Mol. Biol. 339: 1179–1189.
Diamant, S., A. P. Ben-Zvi, B. Bukau, and P. Goloubinoff (2000) Size-dependent disaggregation for stable protein aggregates by the DnaK chaperone machinery.J. Biol. Chem. 275: 21107–21113.
Gragerov, A., E. Nudler, N. Komissarova, G. A. Gaitanaris, M. E. Gottesman, and V. Nikiforov (1992) Cooperation of GroEL/GroES and DnaK/DnaJ heat shock preteins in preventing protein misfolding inEscherichia coli.Proc. Natl. Acad. Sci. USA. 89: 10341–10344.
Szabo, A., T. Langer, H. Schroder, J. Flanagan, B. Bukau, and F. U. Hartl (1994) The ATP hydrolysis-dependent reaction cycle of theEscherichia coli Hsp70 system-DnaK, DnaJ, and GrpE.Proc. Natl. Acad. Sci. USA 91: 10545–10349.
Weissman, J. S., H. S. Rye, W. A. Fenton, J. M. Beechem, and A. L. Horwich (1996) Characterization of the active intermediate of a GroEL-GroES-mediated protein folding reaction.Cell 84: 481–490.
Ying, B. W., H. Taguchi, H. Ueda, and T. Ueda (2004) Chaperone-assisted folding of a single-chain antibody in a reconstituted translation system.Biochem. Biophys. Res. Commun. 320: 1359–1364.
Nishihara, K., M. Kanemori, M. Kitagawa, H. Yanagi. and T. Yura (1998) Chaperone coexpression plasmids: differential and synergistic roles of DnaK-DnaJ-GrpE and GroEL-GroES in assisting folding of an allergen of Japanese cedar pollen Cryj2, inEscherichia coli Appl. Environ. Microbiol. 64: 1694–1699.
Yoon, H. J., Y. J. Choi, O. Miyake, W. Hashimoto, K. Murata, and B. Mikami (2001) Effect of Hisl 92 mutation on the activity of alginate lyase Al-III fromSphingontonas species AI.J. Microbiol. Biotechnol. 11: 118–123.
Hicks, S. J. and P. Gacesa (1996) Heterologous expression of full-length and truncated forms of the recombinant guluronate-specific alginate lyase ofKlebsiella pneumoniae.Enzyme Microb. Technol. 19: 68–75.
Gonzalez-Montalban, N., M. M. Carrio, S. Cuatrecasas, A. Aris, and A. Villaverde (2005) Bacterial inclusion bodies are cytotoxicin vivo in absence of functional chaperones DnaK or GroEL.J. Biotechnol. 118: 406–412.
Park, S. L., E. J. Shin, S. P. Hong, S. J. Jeon, and S. W. Nam (2004) Production of soluble human granulocyte colony stimulating factor inE coli by molecular chaperones.J. Microbiol. Biotechnol. 14: 216–219.
Chen, Y., J. Song, S. F. Sui, and D. N. Wang (2003) DnaK and DnaJ facilitated the folding process and reduced inclusion body formation of magnesium transporter CorA overexpressed inEscherichia coli.Protein Expr. Purif 32: 221–231.
Kwak, Y. H., S. J. Kim, K. Y. Lee, and H. B. Kim (2000) Stress responses of theEscherichia coli groE promoter.J. Microbiol. Biotechnol. 10: 63–68.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, EJ., Park, SL., Jeon, SJ. et al. Effect of molecular chaperones on the soluble expression of alginate lyase inE. coli . Biotechnol. Bioprocess Eng. 11, 414–419 (2006). https://doi.org/10.1007/BF02932308
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02932308