Abstract
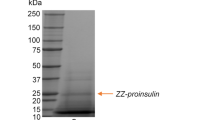
For the production and purification of a single chain human insulin precursor four types of fusion peptides β-galactosidase (LacZ), maltose binding protein (MBP), glutathione-S-transferase (GST), and (His)6-tagged sequence (HTS) were investigated. RecombinantE. coli harboring hybrid genes was cultivated at 37°C for 1 h, and gene induction occurred when 0.2 mM of isopropyl-D-thiogalactoside (IPTG) was added to the culture broth, except forE. coli BL21 (DE3) pLysS harboring a pET-BA cultivation with 1.0 mM IPTG, followed by a longer than 4 h batch fermentation respectively. DEAE-Sphacel and Sephadex G-200 gel filtration chromatography, amylose affinity chromatography, glutathione-sepharose 4B affinity chromatography, and a nickel chelating affinity chromatography system as a kind of immobilized metal ion affinity chromatography (IMAC) were all employed for the purification of a single chain human insulin precursor. The recovery yields of the HTS-fused, GST-fused, MBP-fused, and LacZ-fused single chain human insulin precursors resulted in 47%, 20%, 20%, and 18% as the total protein amounts respectively. These results show that a higher recovery yield of the finally purified recombinant peptides was achieved when affinity column chromatography was employed and when the fused peptide had a smaller molecular weight. In addition the pET expression system gave the highest productivity of a fused insulin precursor due to a two-step regulation of the gene expression, and the HTS-fused system provided the highest recovery of a fused insulin precursor based on a simple and specific separation using the IMAC technique
Similar content being viewed by others
References
Chang, S. G., D. Y. Kim, K. D. Choi, J. M. Shin, and H. C. Shin (1998) Human insulin production from a novel mini-proinsulin which has high receptor-binding activity.Biochem. J. 329: 631–635.
Bae, C. S., M. S. Hong, S. G. Chang, D. Y. Kim, and H. C. Shin (1997) Optimization of fusion proinsulin production by high cell-density fermentation of recombinantE. coli.Biotechnol. Bioprocess Eng. 2: 27–32.
Michael, R. L. and K. L. Kohlmann (1992) Recombinant human Insulin.Biotechnol. Prog. 8: 469–478.
Daniel, C. H., R. R. Browsher, R. L. Brunelle, and J. R. Woodworth (1994) [Lys(B28), Pro (B29)]-Human insulin. A rapidly absorbed analogue of human in sulin.J. Diabetes 43: 396–402.
Demetri, P., E. Sapidou, and J. Caladranis (1995) Computer-aided process analysis and economic evaluation for biosynthetic human insulin production-A case study.Biotechnol. Bioeng. 48: 529–541.
Lee, S. Y., J. H. Ko, M. H. Choi, and D. H. Nam (1996) Fermentation and purification of LacZ-fused single chain insulin precursor for (B30-Homoserine) human insulin.Biotechnol. Bioprocess Eng. 1: 9–12.
Wood, T. K. and S. W. Peretti (1991) Effect of chemically-induced, cloned gene expression inE. coli.Biotechnol. Bioeng. 38: 397–412.
Miao, F. and D. S. Kompala (1992) Over expression of cloned genes using recombinantE. coli regulated by a T7 promoter. 1. Batch cultures and kinetic modeling.Biotechnol. Bioeng. 40: 787–796.
Nam, D. H., J. H. Ko, and S. Y. Lee (1993) Design and cloning of the a novel insulin analogue, (B30-homoserine) human insulin.Arch. Pharm. Res. 16: 271–275.
Kim, S. W. (1995)Expression and purification of B 30-homoscrine human insulin analogue in Escherichia coli. M. S. Thesis, Yeungnam Univ., Daegu, Korea.
Lee, J. S. (1998)Fermentation of recombinant insulin analogue in Escherichia coli and its purification by affinity chromatography. M. S. Thesis, Yeungnam Univ., Daegu, Korea.
Kim, J. Y. and D. D. Y. Ryu (1999) Physiological and environmental effects on metabolic flux change caused by heterologous gene expression inEscherichia coli.Biotechnol. Bioprocess Eng. 4: 170–175.
Marston, F. A. O. (1986) The purification of eucaryotic polypeptides synthesized inEscherichia coli.Biochem. J. 240: 1–12.
Marston, F. A. O. (1987)DNA Cloning, A Practical Approach. vol. 3, pp. 59–88. In: D. M. Clover (ed.) IRL Press, Oxford, UK.
Daniel, M. B. and S. J. Edelstein. (1991)Protein methods, pp. 50–59, Wiley-Liss,
Bradford, M. M. (1976) A rapid and sensitive methods for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding.Anal. Biochem. 72: 248–254.
Grigority, C., J. Hopp, and P. Nelson (1999) Immobilized metal ion affinity chromatography on Co2+-carboxymethylaspartate-agarose superflow, as demonstrate by one-step purification of lactate dehydrogenase from chicken breast muscle.Biotechnol. Appl. Biochem. 29: 19–24.
Müler, K. M., K. M. Arndt, K. Bauer, and A. Plükthun (1998) Tandem immobilized metal-ion affinity chromatography/immunoaffinity purification of His-tagged proteins-evaluation of two anti-His-tag monoclonal antibodies.Anal. Biochem. 259: 54–61.
Vunnum, S., S. Gallant, and S. Cramer (1996) Immobilized metal affinity chromatography: displacer characteristics of traditional mobile phase modifiers.Biotechnol. Prog. 12: 84–91.
Cayuela, C., K. Kai, S. Iijima, and T. Kobayashi (1992) Insecticide production by recombinantB. subtilus 1A96 in fed-batch culture with control of glucose concentration.J. Ferment. Bioeng. 75: 383–386.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, C.W., Park, S.H. & Nam, D.H. Production and purification of single chain human insulin precursors with various fusion peptides. Biotechnol. Bioprocess Eng. 6, 144–149 (2001). https://doi.org/10.1007/BF02931961
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02931961