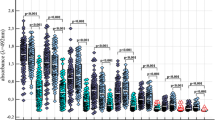
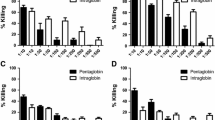
Abstract
TenE. coli K1 strains isolated from the urine of children with urinary tract infections were sensitive to the bactericidal action of normal human serum (NHS). The role of the particular mechanisms of complement activation was determined in the process of killing these strains, showing variable sensitivity to the bactericidal action of NHS; three mechanisms of activation of human complement were observed. Important role of alternative pathway activation in the bactericidal action of NHS againstE. coli K1 strains independent of the classical and lectin pathways was not established.
Similar content being viewed by others
Abbreviations
- EGTA:
-
glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- NeuNAc:
-
5-acetamido-3,5-dideoxy-d-glycero-d-galacto-2-nonulosonic acid
- HS50°C:
-
human serum with inhibited alternative pathway of complement activation
- HSMgEGTA:
-
human serum with inhibited classical and lectin pathways of complement activation
- LPS:
-
lipopolysaccharide(s)
- NHS:
-
normal human serum
- OM:
-
outer membrane
References
Bliss J.M., Silver R.P.: Coating the surface: a model for expression of capsular polysialic acid inEscherichia coli K1.Mol.Microbiol. 21, 221–231 (1996).
Bliss J.M., Garon C.F., Silver R.P.: Polysialic acid export inEscherichia coli K1: the role of KpsT, the ATP-binding component of an ABC transporter, in chain translocation.Glycobiology 6, 445–452 (1996).
Cisowska A., Jankowski S.: The sensitivity ofEscherichia coli strains with K1 surface antigen and rods without this antigen to the bactericidal effect of serum.Folia Microbiol. 49, 471–478 (2004).
Cisowska A., Bugla-Ploskońska G., Tichaczek-Goska D., Doroszkiewicz W., Jankowski S.: The susceptibility ofEscherichia coli strains with sialic acid-containing lipopolysaccharides or capsules to the bactericidal action of normal human serum, pp. 41–47 inProc. 7th Conf. Molecular Biology in Diagnostics of Infectious Diseases and Biotechnology. Publishing House “Wydawnictwo SGGW”, Warsaw 2004.
Cisowska A., Bugla-Płoskońska G., Gamian A., Doroszkiewicz W., Jankowski S.: Relationship between susceptibility to bactericidal action of serum and outer membrane protein patterns inE. coli K1 strains.Pol.J.Environ.Stud. 14, 476–482 (2005).
Devine D.A., Roberts A.P., Rowe B.: Simple technique for detecting K1 antigen ofEscherichia coli.J.Clin.Pathol. 43, 76–78 (1990).
Devine D.A., Roberts A.P.: K1, K5 and O antigens ofEscherichia coli in relation to serum killingvia the classical and alternative complement pathways.J.Med.Microbiol. 41, 139–144 (1994).
Edinger D., Bello E., Mates A.: The heterocytotoxicity of human serum — I. Activation of the alternative complement pathway by heterologous target cells.Cell.Immunol. 29, 174–186 (1977).
Falkenhagen V., Zingler G., Nauman G.: Serum resistance in different serotypes ofEscherichia coli.Zbl.Bacteriol. 275, 216–219 (1991).
Fearon D.T.: Regulation by membrane sialic acid of β1H-dependent decay dissociation of amplification C3 convertase of the alternative complement pathway.Proc.Nat.Acad.Sci.USA 75, 1971–1975 (1978).
Fine D.P., Marney S.R., Colley D.G., Sergent J.S., Des Prez R.M.: C3 shunt activation in human serum chelated with EGTA.J.Immunol. 109, 807–809 (1972).
Franiczek R., Jankowski S.: Synergistic effect of cefotaxime and normal human serum on bactericidal activity against urinary strains ofEscherichia coli with capsular antigen K1.Acta Microbiol.Pol. 3–4, 243–250 (1993).
Frank M.M.: The mechanism by which microorganisms avoid complement attack.Curr.Opin.Immunol. 4, 14–19 (1992).
Frank M.M.: Complement deficiencies.Pediatr.Clinics N.Am. 47, 1339–1354 (2000).
Gamian A., Jones C., Lipiński T., Korzeniowska-Kowal A., Ravenscroft N.: Structure of sialic acid-containing O-specific poly-saccharide fromSalmonella enterica serovar Toucra O48 lipopolysaccharide.Eur.J.Biochem. 267, 3160–3166 (2000).
Johnson J.R.: Virulence factors inEscherichia coli urinary tract infection.Clin.Microbiol.Rev. 4, 80–128 (1991).
Kim K.S.:Escherichia coli translocation at the blood-brain barrier.Infect.Immun. 69, 5217–5222 (2001).
Kahler C.M., Martin L.F., Shih G.C., Rahman M.M., Carlson R.W., Stephens D.S.: The u(2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup BNeisseria meningitidis to resist the bactericidal activity of normal human serum.Infect.Immun. 66, 5939–5947 (1998).
Leying H., Suerbaum S., Kroll H.-P., Stahl D., Opferkuch W.: The capsular polysaccharide is a major determinant of serum resistance in K1-positive blood culture isolates ofEscherichia coli.Infect.Immun. 58, 222–227 (1990).
Marques M.B., Kasper D.L., Pangburn M.K., Wessels M.R.: Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci.Infect.Immun. 60, 3986–3993 (1992).
Meri S., Pangburn M.K.: Discrimination between activators and nonactivators of the alternative pathway of complement: regulationvia a sialic acid/polyanion binding site on factor H.Proc.Nat.Acad.Sci.USA 87, 3982–3986 (1990).
Ram S., MacKinnon F.G., Gulati S., McQuillen D.P., Vogel U., Frosch M., Elkins C., Guttormsen H.-K., Wetzler L.M., Oppermann M., Pangburn M.K., Rice P.A.: The contrasting mechanisms of serum resistance ofNeisseria gonorrhoeae and group BNeisseria meningitidis.Mol.Immunol. 36, 915–928 (1999).
Rautemaa R., Meri S.: Complement-resistance mechanisms of bacteria.Microb.Infect. 1, 785–794 (1999).
Rozenberg-Arska M., Porsius J.C., Jaarsma E.Y., Verhoef J.: Bactericidal, bacteriolytic and opsonic activity of human serum againstEscherichia coli.J.Med.Microbiol. 22, 143–149 (1986).
Vimr E.R., Kalivoda K.A., Deszo E.L., Steenbergen S.M.: Diversity of microbial sialic acid metabolism.Microb.Molec.Biol.Rev. 68, 132–153 (2004).
Vogel U., Claus H., Heinze G., Frosch M.: Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates.Infect.Immun. 67, 954–957 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bugla-Płoskońska, G., Cisowska, A., Karpińska, K. et al. The mechanisms of activation of normal human serum complement byEscherichia coli strains with K1 surface antigen. Folia Microbiol 51, 627–632 (2006). https://doi.org/10.1007/BF02931630
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02931630