Abstract
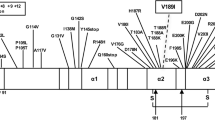
The definitive diagnosis of the CJD (Creutzfeldt-Jakob disease; very rare neurodegenerative disorder) can be established only on the basis of post-mortem examination of the central nervous system tissue. Formaldehyde-fixed paraffin-embedded (FFPE) tissue samples may thus constitute the only material available for molecular pathology analyses. We performed post-mortem analysis of the coding region of the prion-protein gene (PRNP)-sequence variations in two definite CJD cases suggestive of genetic form. Only FFPE tissues were available for molecular analyses. ThePRNP gene open reading frame was amplified from the genomic DNA (FFPE isolated) in four overlapping, two round semi-nested PCR products that were directly sequenced. We found known pathogenic sequence variation g.532 G>A (Asp178Asn) in patient1 but we did not find any pathogenic sequence variation in patient2 despite her origin from the Slovak Orava region. Based on these results, we were able to discriminate between genetic and sporadic form of CJD in patient1 and2, respectively. The established method was found to be efficient for the sequence-variation analysis of the entirePRNP gene coding region using the genomic DNA isolated from the FFPE tissues; it can be employed in other retrospective molecular studies.
Similar content being viewed by others
Abbreviations
- CJD:
-
Creutzfeldt Jacob disease
- FFPE:
-
formaldehyde-fixed paraffin-embedded
- NBF:
-
neutral buffered aqueous formaldehyde
- ORF:
-
open reading frame
- PCR:
-
polymerase chain reaction
- PRNP :
-
prion protein gene
- PrPres:
-
prion protein proteinase-resistant
- SAF:
-
scrappie-associated fibril
- TSE:
-
transmissible spongiform encephalopathy(ies)
References
Alperovitch A., Zerr I., Pocchiari M., Mitrova E., de Pedro Cuesta J., Hegyi I., Collins S., Kretzschmar H., van Duijn C., Will R. G.: Codon 129 prion protein genotype and sporadic Creutzfeldt-Jakob disease.Lancet 353, 1673–1674 (1999).
Barnes W.M.: The fidelity of Taq polymerase catalyzing PCR is improved by an N-terminal deletion.Gene 112, 29–35 (1992).
Barnes W.M.: PCR amplification of up to 35-kb DNA with high fidelity and high yield from λ bacteriophage templates.Proc.Nat.Acad.Sci.USA 91, 2216–2220 (1994).
Bell J.E., Gentleman S.M., Ironside J.W., McCardle L., Lantos P.L., Doey L., Lowe J., Fergusson J., Luthert P., McQuaid S., Allen I.V.: Prion protein immunocytochemistry — UK five center consensus report.Neuropathol.Appl.Neurobiol. 23, 26–35 (1997).
Brown P., Cervenakova L., Boellaard J.W., Stavrou D., Goldfarb L.G., Gajdusek D.C.: Identification of aPRNP gene mutation in Jakob’s original Creutzfeldt-Jakob disease family.Lancet 344, 130–131 (1994).
Budka H., Aguzzi A., Brown P., Brucher J.M., Bugiani O., Gullotta F., Haltia M., Hauw J.J., Ironside J.W., Jellinger K.: Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases).Brain Pathol. 5, 459–466 (1995).
Coombs N.J., Gough A.C., Primrose J.N.: Optimization of DNA and RNA extraction from archival formalin-fixed tissue.Nucl.Acids Res. 27, e12 (1999).
den Dunnen J.T., Antonarakis S.E.: Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion.Human Mutat. 15, 7–12 (2000).
den Dunnen J.T., Antonarakis S.E.: Nomenclature for the description of human sequence variations.Human Genet. 109, 121–124 (2001).
Eckert K.A., Kunkel T.A.: DNA polymerase fidelity and the polymerase chain reaction.PCR Meth.Appl. 1, 17–24 (1991).
Hayashi K.: Manipulation of DNA by PCR, pp. 3–13 in K.B. Mullis, F. Ferre, R.A. Gibbs (Eds):The Polymerase Chain Reaction. Birkhäuser, Boston 1994.
Hill A.F., Joiner S., Wadsworth J.D., Sidle K.C., Bell J.E., Budka H., Ironside J.W., Collinge J.: Molecular classification of sporadic Creutzfeldt-Jakob disease.Brain 126, 1333–1346 (2003).
Howe J.R., Klimstra D.S., Cordon-Cardo C.: DNA extraction from paraffin-embedded tissues using a salting-out procedure: a reliable method for PCR amplification of archival material.Histol.Histopathol. 12, 595–601 (1997).
Isola J., DeVries S., Chu L., Ghazvini S., Waldman F.: Analysis of changes in DNA sequence copy number by comparative genomic hybridization in archival paraffin-embedded tumor samples.Am.J.Pathol. 145, 1301–1308 (1994).
Jackson D.P., Lewis F.A., Taylor G.R., Boylston A.W., Quirke P.: Tissue extraction of DNA and RNA and analysis by the polymerase chain reaction.J.Clin.Pathol. 43, 499–504 (1990).
Keohavong P., Ling L., Dias C., Thilly W.G.: Predominant mutations induced by theThermococcus litoralis, vent DNA polymerase during DNA amplificationin vitro.PCR Meth.Appl. 2, 288–292 (1993).
Kovacs G.G., Trabattoni G., Hainfellner J.A., Ironside J.W., Knight R.S., Budka H.: Mutations of the prion protein gene phenotypic spectrum.J.Neurol. 249, 1567–1582 (2002).
Krawczak M., Reiss J., Schmidtke J., Rosler U.: Polymerase chain reaction: replication errors and reliability of gene diagnosis.Nucl.Acids Res. 17, 2197–2201 (1989).
Kretzschmar H.A., Neumann M., Stavrou D.: Codon 178 mutation of the human prion protein gene in a German family (Backer family): sequencing data from 72-year-old celloidin-embedded brain tissue.Acta Neuropathol.(Berlin) 89, 96–98 (1995).
Lee H.S., Sambuughin N., Cervenakova L., Chapman J., Pocchiari M., Litvak S., Qi H.Y., Budka H., del Ser T., Furukawa H., Brown P., Gajdusek D.C., Long J.C., Korczyn A.D., Goldfarb L.G.: Ancestral origins and worldwide distribution of thePRNP 200K mutation causing familial Creutzfeldt-Jakob disease.Am.J.Human Genet. 64, 1063–1070 (1999).
Mead S., Mahal S.P., Beck J., Campbell T., Farrall M., Fisher E., Collinge J.: Sporadic — but not variant — Creutzfeldt-Jakob disease is associated with polymorphisms upstream ofPRNP exon 1.Am.J.Human Genet. 69, 1225–1235 (2001).
Paabo S., Irwin D.M., Wilson A.C.: DNA damage promotes jumping between templates during enzymatic amplification.J.Biol.Chem. 265, 4718–4721 (1990).
Puckett C., Concannon P., Casby C., Hood L.: Genontic structure of the human prion protein gene.Am.J.Human Genet. 49, 320–329 (1991).
Quach N., Goodman M.F., Shibata D.:In vitro mutation artifacts after formalin fixation and error prone translesion synthesis during PCR.BMC Clin.Pathol. 4, 1 (2004).
Soldevila M., Calafell F., Andres A.M., Yague J., Helgason A., Stefansson K., Bertranpetit J.: Prion susceptibility and protective alleles exhibit marked geographic differences.Human Mutat. 22, 104–105 (2003).
Srinivasan M., Sedmak D., Jewell S.: Effect of fixatives and tissue processing on the content and integrity of nucleic acids.Am.J.Pathol. 161, 1961–1971 (2002).
WHO: WHO Manual for Surveillance of Human Transmissible Spongiform Encephalopathies Including Variant Creutzfeldt-Jakob Disease. WHO, Geneva (Switzerland) 2003; http://www.who.int/bloodproducts/TSE-manual2003.pdf.
Williams C., Ponten F., Moberg C., Soderkvist P., Uhlen M., Ponten J., Sitbon G., Lundeberg J.: A high frequency of sequence alterations is due to formalin fixation of archival specimens.Am.J.Pathol. 155, 1467–1471 (1999).
Wong C., DiCioccio R.A., Allen H.J., Werness B.A., Piver M.S.: Mutations in BRCA1 from fixed, paraffin-embedded tissue can be artifacts of preservation.Cancer Genet.Cytogenet. 107, 21–27 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was funded by the research projects of theMinistry of Education, Youth and Sports of the Czech Republic (no. 111 100 003 and 002 162 0806).
Rights and permissions
About this article
Cite this article
Sikora, J., Srbová, A., Koukolík, F. et al. Retrospective sequence analysis of the humanPRNP gene from the formaldehyde-fixed paraffin-embedded tissues: Report of two cases of creutzfeldt-jakob disease. Folia Microbiol 51, 619–625 (2006). https://doi.org/10.1007/BF02931629
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02931629