Abstract
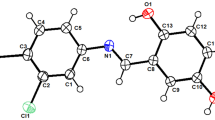
2-(2-Pyridinyl)-(L I), 2-(6-methyl-2-pyridinyl)-(L II), 2-(6-methyl-2-pyridinyl)-5-methyl-(L III), 2-(3-pyridinyl)- (L IV), 2-(3-pyridinyl)-5-methyl-1H-benzimidazoles (L V) and their complexes with Fe(NO3)3, Cu(NO3)2, Zn(NO3)2, and AgNO3 were synthesized and antibacterial activity of the compounds was tested towardStaphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, Shigella flexneri, Proteus mirabilis and antifungal activity againstCandida albicans. The methyl groups ofL III increase the antimicrobial activity. The AgI complexes have considerable activity toward the microorganisms. Some ZnII complexes show an antimicrobial effect againstS. aureus andS. flexneri, although the ligands themselves have no effect. CuII complexes have a considerable antibacterial effect toS. aureus andS. epidermidis.
Similar content being viewed by others
References
Biradar N.S., Goudar T.R.: Addition compounds of niobium(V) with 2-substituted benzimidazoles.J.Inorg.Nucl.Chem. 39, 358–360 (1977)
Clark R.B., Ferreira S.H., Mehta N.B., Thorogodd P.B., Vinegar R.: Imidazole or benzimidazole derivative containing anti-inflammatory preparations.German Pat. 2 634 409 (1975);C.A. 86, 161 312u (1977).
Foks H., Janowiec M.: Pyrazine derivatives — IX. Synthesis and tuberculostatic activity of 2-pyrazinylbenzimidazoles.Acta Polon. Pharm. 35, 281–288 (1978);C.A. 90, 168 536m (1978).
Gupta S.K., Mishra L.K.: Oxovanadium(IV) complexes with 2-(o-hydroxyphenyl)benzimidazole and related ligands.J.Inorg.Nucl.Chem. 41, 890–891 (1979).
Hisano T., Ichikawa M., Tsumoto K., Tasaki M.: Synthesis of benzoaxazoles, benzothiazoles and benzimidazoles and evaluation of their antifungal, insecticidal and herbicidal activities.Chem.Pharm.Bull. 30, 2996–3004 (1982).
Ichikawa M., Nabeya S., Muraoka K., Hisano T.: Acidic properties of benzimidazoles and substituent effects — IV. Relationship between the acidities ofN′-(substituted phenyl)arylamidines and ring closures to imidazole.Chem.Pharm.Bull. 27, 1255–1264 (1979).
Kharizanova T., Torlakov I., Zhelyankov L., Todorova N., Sheikov N.: Antinematodal activity of 2-substituted benzimidazoles.Tr.Nauchnoized.Khim.-Farm.Inst. 8, 347–352 (1972);C.A. 79, 38 526v (1972).
Konovalova L.M., Ozeretskovskaya N.N., Kolosova M.O.: Search for specific therapy of trichinosis — III. Derivatives of benzimidazole, 2-(3-pyridyl)benzimidazole and 2-(m-nitrophenyl)benzimidazole, in experimental trichinellosis in white mice.Med.Parazitol.Parazit.Bolez. 35, 551–556 (1966);C.A. 66, 84 553f (1967).
Küçükbay H., Durmaz B.: Antifungal activity of organic and organometallic derivatives of benzimidazole and benzothiazole.Arzneim.Forsch. 47, 667–670 (1997).
Lane T.J., Nakagawa I., Walter J.L., Kandathil A.J.: Infrared investigation of certain imidazole derivatives and their metal chelates.Inorg.Chem. 1, 267–276 (1962).
National Committee for Clinical Laboratory Standards: Methods for broth dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M7-A4. NCCLS, Villanova (USA) 1997a.
National Committee for Clinical Laboratory Standards: Reference method for dilution antifungal susceptibility tests of yeasts. Approved Standard M27-A. NCCLS, Villanova (USA) 1997b.
Nose T., Kono T., Yoshino K., Ito K., Kagaya H., Tsukamoto G.: Inflammation inhibitors. Anti-inflammatory pyridylbenzimidazoles.Japan Kokai Tokyo Koho (Japan.Pat.) 79 95 734 (1979);C.A. 92, P82 430b (1980).
Tavman A., Ülkuseven B.: Studies of metal complexes of 2-(2-pyridinyl)-, 2-(6-methyl-2-pyridinyl)-, 2-(3-pyridinyl)-1H-benzimidazoles with some d8–10 ions.Synth.React.Inorg.Met.-Org.Chem. 29, 1805–1819 (1999).
Ülküseven B., Tavman A., Ötük G.: Synthesis, characterization and antimicrobial activity of d8–10 metal complexes of some 2-substituted-1H-benzimidazoles.Metal-Based Drugs 6, 63–67 (1999).
Ülküseven B., Tavman A., Ötük G., Birteksöz S.: Antimicrobial activity of FeIII, CuII, AgI, ZnII and HgII complexes of 2-(2-hydroxy-5-bromo/nitro-phenyl)-1H- and 2-(2-hydroxyphenyl)-5-methyl/chloro/nitro-1H-benzimidazeles.Folia Microbiol. 47, 481–487 (2002).
Author information
Authors and Affiliations
Additional information
This work was supported by the Research Fund ofThe University of Istanbul, project no. 1087/010598.
Rights and permissions
About this article
Cite this article
Tavman, A., Ülküseven, B., Birteksöz, S. et al. Antimicrobial activity of some 2- and 3-pyridinyl-1H-benzimidazoles and their FeIII, CuII, ZnII, and AgI complexes. Folia Microbiol 48, 479–483 (2003). https://doi.org/10.1007/BF02931328
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02931328