Abstract
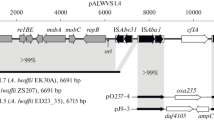
Three strains ofEscherichia fergusonii (EF873, EF1496, EF939) of 50 strains tested produced the hydroxamate siderophore aerobactin. Screening of a cosmid library of the strain EF873 chromosomal DNA (in aerobactin nonproducingEscherichia coli VCS257) for aerobactin production identifiediucABCD andiutA gene orthologues. The predicted IucABCD and IutA proteins showed 59–65% identity to the corresponding proteins ofShigella flexneri andE. coli. Aerobactin molecules synthesized byE. fergusonii andE. coli strains stimulated growth of aerobactin indicator strains harboring eitherE. coli orE. fergusonii iutA genes. In the 12 kb upstream and 17 kb downstream regions of theiuc andiut genes, 20 additional ORFs were identified. Their gene products showed homology to proteins fromE. coli, S. flexneri, Klebsiella aerogenes, Pseudomonas aeruginosa andVibrio cholerae. Probes recognizing DNA sequences from a region of more than 25 kb, which included theiucABCD andiutA genes, hybridized with chromosomal DNA of two aerobactin-producing strains (EF873 and EF939), but not with other nonproducingE. fergusonii strains tested. These data, together with the genetic organization of this region, suggest thatE. fergusonii iucABCD iutA genes are a portion of a larger segment of DNA similar to pathogenicity islands of other bacteria.
Similar content being viewed by others
References
Bain M.S., Green C.C.: Isolation ofEscherichia fergusonii in cases clinically suggestive of salmonellosis.Vet.Rec. 144, 511 (1999).
Blattner F.R., Plunkett G. 3rd,Bloch C.A., Perna N.T., Burland V., Riley M., Collado-Vides J., Glasner J.D., Rode C.K., Mayhew G.F., Gregor J., Davis N.W., Kirkpatrick H.A., Goeden M.A., Rose D.J., Mau B., Shao Y.: The complete genome sequence ofEscherichia coli K-12.Science 277, 1453–1474 (1997).
Braun V., Gross R., Koster W., Zimmermann I.: Plasmid and chromosomal mutants in the iron(III)-aerobactin transport system ofEscherichia coli. Use of streptonigrin for selection.Mol.Gen.Genet. 192, 131–139 (1983).
Burland V., Plunkett G. 3rd,Sofia H.J., Daniels D.L., Blattner F.R.: Analysis of theEscherichia coli genome VI: DNA sequence of the region from 92.8 through 100 minutes.Nucl.Acids.Res. 23, 2105–2119 (1995).
Chaudhury A., Nath G., Tikoo A., Sanyal S.C.: Enteropathogenicity and antimicrobial susceptibility of newEscherichia spp.J.Diarrhoeal Dis.Res. 17, 85–87 (1999).
Farmer J.J. 3rd,Fanning G.R., Davis B.R., O’Hara C.M., Riddle C., Hickman-Brenner F.W., Asbury M.A., Lowery V.A. 3rd,Brenner D.J.:Escherichia fergusonii andEnterobacter taylorae, two new species ofEnterobacteriaceae isolated from clinical specimens.J.Clin.Microbiol. 21, 77–81 (1985).
Funke G., Hany A., Altwegg M.: Isolation ofEscherichia fergusonii from four different sites in a patient with pancreatic carcinoma and cholangiosepsis.J.Clin.Microbiol. 31, 2201–2203 (1993).
Furuya N., Komano T.: Determination of the nick site atoriT of IncI1 plasmid R64: global similarity oforiT structures of IncI1 and IncP plasmids.J.Bacteriol. 173, 6612–6617 (1991).
Gilis A., Khan M.A., Cornelis P., Meyer J.M., Mergeay M., van der Lelie D.: Siderophore-mediated iron uptake inAlcaligenes eutrophus CH34 and identification ofaleB encoding the ferric iron-alcaligin E receptor.J.Bacteriol. 178, 5499–5507 (1996).
Hacker J., Blum-Oehler G., Muhldorfer I., Tschape H.: Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution.Mol.Microbiol. 23, 1089–1097 (1997).
Hacker J., Kaper J.B.: Pathogenicity islands and the evolution of microbes.Ann.Rev.Microbiol. 54, 641–679 (2000).
Heidelberg J.F., Eisen J.A., Nelson W.C., Clayton R.A., Gwinn M.L., Dodson R.J., Haft D.H., Hickey E.K., Peterson J.D., Umayam L., Gill S.R., Nelson K.E., Read T.D., Tettelin H., Richardson D., Ermolaeva M.D., Vamathevan J., Bass S., Qin H., Dragoi I., Sellers P., McDonald L., Utterback T., Fleishmann R.D., Nierman W.C., White O.: DNA sequence of both chromosomes of the cholera pathogenVibro cholerae.Nature 406, 477–483 (2000).
Herrero M., de Lorenzo V., Neilands J.B.: Nucleotide sequence of theiucD gene of the pColV-K30 aerobactin operon and topology of its product studied withphoA andlacZ gene fusions.J.Bacteriol. 170, 56–64 (1988).
Kaneko T., Sato S., Kotani H., Tanaka A., Asamizu, E., Nakamura Y., Miyajima N., Hirosawa M., Sugiura M., Sasamoto S., Kimura T., Hosouchi T., Matsuno A., Muraki A., Nakazaki N., Naruo K., Okumura S., Shimpo S., Takeuchi C., Wada T., Watanabe A., Yamada M., Yasuda M., Tabata S.: Sequence analysis of the genome of the unicellular cyanobacteriumSynechocystis sp. strain PCC6803 — II. Sequence determination of the entire genome and assignment of potential protein-coding regions.DNA Res. 30, 109–136 (1996).
Keller R., Pedroso M.Z., Ritchmann R., Silva R.M.: Occurrence of virulence-associated properties inEnterobacter cloacae.Infect.Immun. 66, 645–649 (1998).
Krone W.J.A., Stegehuis F., Koningstein G., van Doorn C., Roosendaal B., de Graaf F.K., Oudega B.: Characterization of the pCoIV-K30 encoded cloacin DF13/aerobactin outer membrane receptor protein ofEscherichia coli: isolation and purification of the protein and analysis of its nucleotide sequence and primary structure.FEMS Microbiol.Lett. 26, 153–161 (1985).
Lawlor K.M., Payne S.M.: Aerobactin genes inShigella spp.J.Bacteriol. 160, 266–272 (1984).
Lawrence J.G., Roth J.R.: The cobalamin (coenzyme B12) biosynthetic genes ofEscherichia coli.J.Bacteriol. 177, 6371–6380 (1995).
Leung H.B., Kvalnes-Krick K.L., Meyer S.L., de Riel J.K., Schramm V.L.: Structure and regulation of the AMP nucleosidase gene (amn) fromEscherichia coli.Biochemistry 28, 8726–8733 (1989).
de Lorenzo V., Bindereif A., Paw B.H., Neilands J.B.: Aerobactin biosynthesis and transport genes of plasmid ColV-K30 inEscherichia coli K-12.J.Bacteriol. 165, 570–578 (1986).
de Lorenzo V., Martinez J.L.: Aerobactin production as a virulence factor: a reevaluation.Eur.J.Clin.Microbiol.Infect.Dis. 7, 621–629 (1988).
Martinez J.L., Herrero M., de Lorenzo V.: The organization of intereistronic regions of the aerobactin operon of pColV-K30 may account for the differential expression of theiucABCD iutA genes.J.Mol.Biol. 238, 288–293 (1994).
Moss J.E., Cardozo T.J., Zychlinsky A., Groisman E.A.: TheselC-associated SHI-2 pathogenicity island ofShigella flexneri.Mol. Microbiol. 33, 74–83 (1999).
Murakami K., Fuse H., Takimura O., Inoue H., Yamaoka Y.: Cloning and characterization of theiutA gene which encodes ferric aerobactin receptor from marineVibrio species.Microbios 101, 137–146 (2000).
Okujo N., Yamamoto S.: Identification of the siderophores fromVibrio hollisae andVibrio mimicus as aerobactin.FEMS Microbiol. Lett. 118, 187–192 (1994).
Perna N.T., Plunkett G. 3rd,Burland V., Mau B., Glasner J.D., Rose D.J., Mayhew G.F., Evans P.S., Gregor J., Kirkpatrick H.A., Posfai G., Hackett J., Klink S., Boutin A., Shao Y., Miller L., Grotbeck E.J., Davis N.W., Lim A., Dimalanta E., Potamousis K., Apodaca J., Anantharaman T.S., Lin J., Yen G., Schwartz D.C., Welch R.A., Blattner F.R.:Blattner F.R.: Genome sequence of enterohaemorrhagicEscherichia coli O157:H7.Nature 409, 529–533 (2001).
Podschun R., Fischer A., Ullmann U.: Characterization ofKlebsiella terrigena strains from humans: hemagglutinins, serum resistance, siderophore synthesis, and serotypes.Epidemiol.Infect. 125, 71–78 (2000).
Postle K., Good R.F.: DNA sequence of theEscherichia coli tonB gene.Proc.Nat.Acad.Sci.USA 80, 5235–5239 (1983).
Riede I., Drexler K., Eschbach M.L., Henning U.: DNA sequence of the tail fiber genes 37, encoding the receptor recognizing part of the fiber, of bacteriophages T2 and K3.J.Mol.Biol. 191, 255–266 (1986).
Sambrook J., Fritsch E.F., Maniatis T.:Molecular Cloning: a Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor (USA) 1989.
Schwacha A., Bender R.A.: Thenac (nitrogen assimilation control) gene fromKlebsiella aerogenes.J.Bacteriol. 175, 2107–2115 (1993).
Šmajs D., Weinstock G.M.: The iron- and temperature-regulatedcjrBC genes ofShigella and enteroinvasiveEscherichia coli strains code for colicin Js uptake.J.Bacteriol. 183, 3958–3966 (2001).
Stover C.K., Pham N.Q., Erwin A.L., Mizoguchi S.D., Warrener P., Hickey M.J., Brinkman F.S., Hufnagle W.O., Kowalik D.J., Lagrou M., Garber R.L., Goltry L., Tolentino E., Westbrock-Wadman S., Yuan Y., Brody L.L., Coulter S.N., Folger K.R., Kas A., Larbig K., Lim R., Smith K., Spencer D., Wong G.K., Wu Z., Paulsen I.T.: Complete genome sequence ofPseudomonas aeruginosa PA01, an opportunistic pathogen.Nature 406, 959–964 (2000).
Valvano M.A., Crosa J.H.: Aerobactin iron transport genes commonly encoded by certain ColV plasmids occur in the chromosome of a human invasive strain ofEscherichia coli K1.Infect.Immun. 46, 159–167 (1984).
Vernet V., Philippon A., Madoulet C., Vistelle R., Jaussaud R., Chippaux C.: Virulence factors (aerobactin and mucoid phenotype) inKlebsiella pneumoniae andEscherichia coli blood culture isolates.FEMS Microbiol.Lett. 130, 51–57 (1995).
Visca P., Filetici E., Anastoasio M.P., Vetriani C., Fantasia M., Orsi N.: Siderophore production bySalmonella species isolated from different sources.FEMS Microbiol.Lett. 63, 225–231 (1991).
Vokes S.A., Reeves S.A., Torres A.G., Payne S.M.: The aerobactin iron transport system genes inShigella flexneri are present within a pathogenicity island.Mol.Microbiol. 33, 63–73 (1999).
Wang K., Boysen C., Shizuya H., Simon M.I., Hood L.: Complete nucleotide sequence of two generations of a bacterial artificial chromosome cloning vector.Biotechniques 23, 992–994 (1997).
Whipp M.J., Camakaris H., Pittard A.J.: Cloning and analysis of theshiA gene, which encodes the shikimate transport system ofEscherichia coli K-12.Gene 209, 185–192 (1998).
Williams P.H.: Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains ofEscherichia coli.Infect.Immun. 26, 925–932 (1979).
Author information
Authors and Affiliations
Additional information
This work was supported by grants from theUS Public Health Service (R01 DE1 2488 and R01 DE1 3759) to the third author, and by the grant of theGrant Agency of the Czech Republic (no. 310/01/0013).
Rights and permissions
About this article
Cite this article
Šmajs, D., Šmarda, J. & Weinstock, G.M. TheEscherichia fergusonii iucABCD iutA genes are located within a larger chromosomal region similar to pathogenicity islands. Folia Microbiol 48, 139–147 (2003). https://doi.org/10.1007/BF02930946
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02930946