Summary
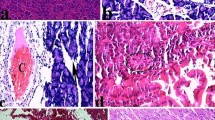
Acute edematous pancreatitis was induced in Wistar male rats by iv infusion of cerulein (CR) in the dose of 5.10-6g.kg-1.h-1 during 3 or 6 h. The effect of BN 52021—platelet activating factor (PAF) receptor antagonist, against this model of disease was examined. BN 52021 was applied iv as a bolus injection in the dose of 5.10-3g.kg-1 at 0 time. Treatment with this agent significantly ameliorates cerulein-induced acute pancreatitis in rats. The effect of BN 52021 was expressed by significant reduction of pancreas edema, diminution of hyperamylasemia, lack of superoxide dismutase activity depletion, and inhibition of lipid peroxidation in pancreatic tissue. These changes were accompanied by significant reduction of acinar cells vacuolization and remarkable inhibition of infiltration with inflammatory cells in the interacinar space. We suppose that beneficial effect of BN 52021 against cerulein-induced acute pancreatitis in rats depends on the prevention of inflammatory cells activation and subsequent generation of oxygen radicals within pancreatic tissue.
Similar content being viewed by others
Referenes
Sanfey H, Bulkley G, Cameron J. The pathogenesis of acute pancreatitis: the role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann. Surg. 1984; 200: 405–413.
Sanfey H, Bulkley G, Cameron J. The pathogenesis of acute pancreatitis: The source and role of oxygen-derived free radicals in three different experimental models. Ann. Surg. 1985; 201: 633–639.
Guice KS, Miller DE, Oldham KT, Townsend CM, Thompson JC. Superoxide dismutase and catalase: a possible role in established pancreatitis. Am. J. Surg. 1986; 151: 163–169.
Wisner JR, Renner IG. Allopurinol attenuates caerulein induced acute pancreatitis in the rat. Gut 1988; 29: 926–929.
Wisner J, Green D, Ferrell L, Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut 1988; 29: 1516–1523.
Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979; 59: 527–605.
Comporti M. Biology of disease. Lipid peroxidation and cellular damage in toxic liver injury. Lab. Invest. 1985; 53: 599–623.
Freeman BA, Crapo JD. Biology of disease. Free radicals and tissue injury. Lab. Invest. 1982; 47: 412–426.
Fridovich I. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 1986; 247: 1–11.
Kappus H, Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia 1981; 37: 1233–1241.
McCord JM, Fridovich I. Superoxide dismutase, an enzymic function for erythrocuprein. J. Biol. Chem. 1969; 244: 6049–6055.
Marklund SL. Extracellular superoxide dismutase in human tissues and human cell lines. J. Clin. Invest. 1984; 74: 1398–1403.
Dabrowski A, Gabryelewicz A, Wereszczyńska-Siemiatkowska U, Chyczewski L. Oxygen- derived free radicals in cerulein-induced acute pancreatitis. Scand. J, Gastroenterol. 1988; 23: 1245–1249.
Benveniste J, Henson PM, Cochrane CG. Leukocyte-dependent histamine release from rabbit platelets. I. The role of IgE, basophils and platelet activating factor. J. Exp. Med. 1972; 136: 1356–1377.
Braquet P. The Ginkgolides: Potent platelet-activating factor antagonists isolated from Ginkgo biloba L. Drugs of the Future 1987; 12: 643–699.
Soling HD, Eibl H, Fest W. Acetylcholine-like effects of l-O-alkyl-2-acetyl-sn-glycero-3- phosphocholine (“platelet-activating factor”) and its analogues in exocrine secretory glands. Eur. J. Biochem. 1984; 144: 65–72.
Emanuelli G, Montrucchio G, Gaia E, Dughera L, Corvetti G, Gubetta L. Experimental acute pancreatitis induced by platelet activating factor in rabbits. Am. J. Pathol. 1989; 134: 315–326.
Janear S, De Giaccobi G, Braquet P. Immune complex-induced pancreatitis. Effect of BN 52021, a selective antagonist of platelet activating factor, In: The Ginkgolides: Chemistry, Biology, Pharmacology and Clinical Sciences. P. Braquet (Ed.). Meth. Find. Exp. Clin. Pharmacol. 1987.
Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch. (A) 1977; 373: 97–117.
Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: The role of lipid peroxidation in cardiac toxicity and tumor responce. Science 1977; 197: 165–167.
Smith JB, Ingerman CB, Silver MJ. Malondiaidehyde formation as an indicator of pro- staglandin production by human platelets. J. Lab. Clin. Med. 1976; 88: 167–172.
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972; 247: 3170–3175.
Sykes JA, McCormack FX, O’Brien TJ. A preliminary study of the superoxide dismutase content of some human tumors. Cancer Res. 1978; 38: 2759–2762.
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951; 193: 265–275.
Caraway WT. A stable starch substrate for the determination of amylase in serum and other body fluids. Am. J. Clin. Path. 1959; 33: 97–108.
Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology 1985; 88: 1192–1204.
Rutledge PL, Saluja AK, Powers RE, Steer ML. Role of oxygen-derived free radicals in diet-induced hemorrhagic pancreatitis in mice. Gastroenterology 1987; 93: 41–47.
Forman HJ, Thomas MJ. Oxidant production and bactericidal activity of phagocytes. Ann. Rev. Physiol. 1986; 48: 669–680.
Suematsu M, Kurose I, Asako H, Miura S, Tsuchiya M. In vivo visualization of oxyradical- dependent photoemission during endothelium-granulocyte interaction in microvascular beds treated with platelet-activating factor. J. Biochem. 1989; 106: 355–360.
Soling HD, Fest W. Synthesis of l-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet activating factor) in exocrine glands and its control by secretagogues. J. Biol. Chem. 1986; 261: 13916–13922.
Gilrane Glover WJ, Granger DN, Holt SL, and Powers RE. A temporal role of neutrophils in the pathogenesis of caerulein-induced acute pancreatitis. Pancreas (abstr.) 1989; 4: 617.
Karges W, Willemer S, Feddersen CO, Adler G. The influence of experimental granulo- cytopenia on cerulein-induced pancreatitis in the rat. Pancreas (abstr.) 1989; 4: 622.
Lankisch PG, Pohl U, Otto J, Rahlf G. When should treatment of acute experimental pancreatitis be started? The early phase of bile induced acute pancreatitis. Res. Exp. Med. 1988; 188: 123–129.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dabrowski, A., Gabryelewicz, A. & Chyczewski, L. The effect of platelet activating factor antagonist (BN 52021) on cerulein-induced acute pancreatitis with reference to oxygen radicals. Int J Pancreatol 8, 1–11 (1991). https://doi.org/10.1007/BF02930218
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02930218