Abstract
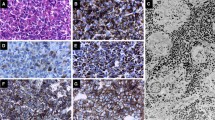
In addition to its structural function, cytokeratin may have other important roles within cells. We have reported that in growth hormone-producing adenomas (GH cell adenomas), two distinct types can be recognized by their cytokeratin distribution patterns (dot-like or perinuclear pattern) and that each type has different clinicopathological and endocrinological properties. To confirm these phenomena in a larger series and to clarify the significance of different cytokeratin distribution patterns, we studied cytokeratin localization in 70 GH cell adenomas from acromegalic patients. Type I adenomas ( 15) almost exclusively (>98%) composed of cells with a prominent, dot-like distribution; type 2 adenomas (36) comprised of cells with perinuclear cytokeratin; and type 3 adenomas (11) comprised of both cell types were separated. The remaining 8 did not exhibit a distinct distribution pattern. By electron microscopic immunocytochemistry for cytokeratin, dot-like distribution corresponded to fibrous bodies, whereas perinuclear distribution represented immune deposition in the perinuclear zone. Immunohistochemistry for GH, prolactin, β-thyrotropin, and α-subunit of glycoprotein hormones revealed a reduced expression of these hormones in type 1 adenomas, compared with types 2 and 3 adenomas. In normal pituitary glands, almost all GH cells showed a perinuclear cytokeratin distribution, and only a few GH cells exhibited a dot-like pattern. These findings suggest that a dot-like cytokeratin distribution in GH cells may be pathological (a change from physiological perinuclear distribution) and that adenomas with such a distribution may reduce endocrine activities as a result of unknown factors.
Similar content being viewed by others
References
Bando H, Sano T, Ohshima T, Zhang CY, Yamasaki R, Matsumoto K, Saito S. Differences in pathological findings and growth hormone responses in patients with growth hormone-producing pituitary adenoma. Endocrinol Jpn 39:355–363, 1992.
Blose ST, Meltzer DI, Feramisco JR. 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J Cell Biol 98:847–858, 1984.
Doorbar J, Ely S, Sterling J, McLean C, Crawford L. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature 352:824–827, 1991.
Fanghanel G, Larraza O, Villalobos M, Fanghanel L, Velasco M, Velasco F. Differential response to aminergic stimuli and biological behavior of growth hormone secreting pituitary adenomas. Can J Neurol Sci 17:78–82, 1990.
Hüfler H, Denk H, Walter GF. Immuno- histochemical demonstration of cytokeratins in endocrine cells of the human pituitary gland and in pituitary adenomas. Virchows Arch [A] 404:359–368, 1984.
Horvath E, Kovacs K. Morphogenesis and significance of fibrous bodies in human pituitary adenomas. Virchows Arch [B] 27:69–78, 1978.
Horvath E, Kovacs K. Fine structural cytology of the adenohypophysis in rat and man. J Electron Microsc Tech 8:401–432, 1988.
Ironside JW, Royds JA, Jefferson AA, Timperley WR. Immunolocalisation of cytokeratins in the normal and neoplastic human pituitary gland. J Neurol Neurosurg Psychiatry 50:57–65, 1987.
Ito K, Osamura Y. Immunohistochemical appearance of “keratin bodies” in the human pituitary GH producing adenomas and their clinicopathological significance. Pathol Clin 8:1175–1181, 1990 (in Japanese).
Kasper M, Stosiek P, van Muijen GNP, Moll R. Cell type heterogeneity of intermediate filament expression in epithelia of the human pituitary gland. Histochemistry 93: 93–103, 1989.
Klymkowsky MW, Miller RH, Lane EB. Morphology, behavior, and interaction of cultured epithelial cells after the antibody-induced disruption of keratin filament organization. J Cell Biol 96:494–509, 1983.
Knapp LW, O’Guin WM, Sawyer RH. Rearrangement of the keratin cytoskeleton after combined treatment with microtubule and microfilament inhibitors. J Cell Biol 97:1788–1794, 1983.
Kovacs K, Horvath E. Pathology of growth hormone-producing tumors of the human pituitary. Semin Diagn Pathol 3:18–33, 1986.
Melmed S. Acromegaly. N Engl J Med 322:966–977, 1990.
Neumann PE, Goldman JE, Horoupian DS, Hess MA. Fibrous bodies in growth hormone-secreting adenomas contain cytokeratin filaments. Arch Pathol Lab Med 109:505–508, 1985.
Riedel M, Saeger W, Ludecke DK. Grading of pituitary adenomas in acromegaly. Comparison of light microscopical, immunocytochemical, and clinical data. Virchows Arch [A] 407:83–95, 1985.
Roy S. Cytoplasmic filamentous masses in chromophobe adenoma of the human pituitary gland. J Pathol 125:151–154, 1978.
Sano T, Ohshima T, Yamada S. Expression of glycoprotein hormones and intracy toplasmic distribution of cytokeratin in growth hormone-producing pituitary adenomas. Pathol Res Pract 187:530–533, 1991.
Sano T. Use of ultrastructural immuno-histochemistry in human pituitary pathology. Microsc Res Tech 20:152–161, 1992.
Scheithauer BW, Horvath E, Kovacs K, Laws ER Jr, Randall RV, Ryan N. Plurihormonal pituitary adenomas. Semin Diagn Pathol 3:69–82, 1986.
Shyy TT, Asch BB, Asch HL. Concurrent collapse of keratin filaments, aggregation of organelles, and inhibition of protein synthesis during the heat shock response in mammary epithelial cells. J Cell Biol 108:997–1008, 1989.
Smedts F, Ramaekers F, Robben H, Pruszczynski M, van Muijen G, Lane B, Leigh I, Vooijs P. Changing patterns of keratin expression during progression of cervical intraepithelial neoplasia. Am J Pathol 136:657–668, 1990.
Trouillas J, Girod C, Lheritier M, Claustrat B, Dubois MP. Morphological and biochemical relationships in 31 human pituitary adenomas with acromegaly. Virchows Arch [A] 389:127–142, 1980.
Trouillas J, Girod C, Loras B, Claustrat B, Sassolas G, Perrin G, Buonaguidi R. The TSH secretion in the human pituitary adenomas. Pathol Res Pract 183:596–600, 1988.
Tsuneyoshi M, Daimaru Y, Hashimoto H, Enjoji M. The existance of rhabdoid cells in specified soft tissue sarcomas. Histopathological, ultrastructural and immunohistochemical evidence. Virchows Arch [A] 411:509–514, 1987.
Weeks DA, Beckwith JB, Mierau GW, Zuppan CW. Renal neoplasms mimicking rhabdoid tumor of kidney. A report from the National Wilms’ Tumor Study Pathology Center. Am J Pathol 15:1042–1054, 1991.
Yamada S, Sano T, Stefaneanu L, Kovacs K, Aiba T, Shishiba Y. Endocrine and morphologic study of a clinically silent somatotroph adenoma of the human pituitary. J Clin Endocrinol Metab 76:352–356, 1993.
Yamada S, Takahashi M, Hara M, Sano T, Aiba T, Shishiba Y. GH and PRL gene expression in human densely granulated and sparsely granulated somatotroph adenomas using in situ hybridization with digoxigenin-labeled probes. Proceedings of 73th Endocrine Society Meeting, Las Vegas, Nevada, 1993 (abstract).
Yamada S, Aiba T, Sano T, Kovacs K, Shishiba Y, Takada K. Gh-producing pituitary adenomas: Correlations between clinical or endocrinological findings and morphology. Neurosurgery 1994 (in press).
Young DG, Bahn RC, Randall RV. Pituitary tumors associated with acromegaly. J Clin Endocr 25:249–259, 1965.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sano, T., Yamada, S., Hi rose, T. et al. Cytokeratin distribution and functional properties of growth hormone-producing pituitary adenomas. Endocr Pathol 5, 107–113 (1994). https://doi.org/10.1007/BF02921378
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02921378