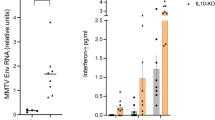
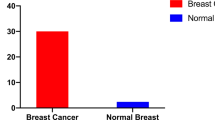
Abstract
Mouse mammary tumor viruses (MMTV) are retroviruses that induce mammary carcinomas. An interesting feature of these viruses is the superantigen (SAg) encoded in an open reading frame within the 3′ long terminal repeat. The mechanism by which ingestion of milk-borne virus results in infection of the host mammary tissue remains incompletely understood. However, a working model has been proposed in which the interaction between viral SAg, T-cell receptor and MHC class II I-E facilitates viral replication and hence infectivity. In this review we summarize current studies demonstrating the role of SAg stimulation in susceptibility to MMTV infection.
Similar content being viewed by others
References
Festenstein H: Immunogenetic and biological aspects of in vitro lymphocyte allotransformation (MLR) in the mouse. Transplant Rev 1973; 15:62–68.
Frankel WN, Rudy C, Coffin JM, Huber BT: Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature 1991; 349:526–528.
Marrack P, Kushnir E, Kappler J: A maternally inherited superantigen encoded by mammary tumour viruses. Nature 1991;349:524–526.
Fleischer B, Hartwig U: T lymphocyte stimulation by microbial superantigens. Chem Immunol 1992;55: 56–64.
Marrack P, Kappler J: The staphylococcal enterotoxins and their relatives. Science 1990;248:705–711.
Tomai MA, Aelion JA, Dockter ME, Majumdar G, Spinella DG, Kolb M: T cell receptor V gene usage by human T cells stimulated with the superantigen streptococcal M protein. J Exp Med 1991;174:285–288.
Hügin AW, Vacchio MS, Morse HC III: A virus-encoded superantigen in a retrovirus-induced immunodeficiency syndrome. Science 1991;252: 424–429.
Kanagawa O, Nussrallah BA, Wiebenga ME, Murphy KM, Morse HC, Carbone FR: Murine AIDS superantigen reactivity of the T cells bearing Vβ5 T cell antigen receptor. J Immunol 1992;149:9–16.
Acha-Orbea H: Retroviral superantigens. Immunology 1992;55:65–86.
Cole BC, Atkin CL: TheMycoplasma arthritidis T-cell mitogen, MAM, a model superantigen. Immunol Today 1991;12:271–275.
Kronenberg M, Siu G, Hood L, Shastri N: The molecular genetics of the T cell antigen receptor and T cell antigen recognition. Annu Rev Immunol 1986;4:529–592.
Fink P, Matis L, McElligot D, Bookman M, Hedrick M: Correlations between T cell specificity and structure of the T cell receptor. Nature 1986;321:219–226.
Davis MM, Bjorkman PJ: T-cell antigen receptor genes and T-cell recognition. Nature 1988;334:395–401.
Abe R, Vacchio MS, Fox B, Hodes RJ: Preferential expression of the T-cell receptor Vβ3 gene by Mlsc reactive T cells. Nature 1988;335:827–830.
Pullen AM, Wade T, Marrack P, Kappler JW: Identification of the region of T cell receptor β chain that interacts with self superantigen Mls-1a. Cell 1988;61:1365–1374.
Choi Y, Herman A, DiGiusto D, Wade T, Marrack P, Kappler J: Residues of the variable region of the T-cell-receptor β-chain that interact withS. aureus toxin superantigen. Nature 1990;346:471–473.
Bellio M, Lone YC, de la Calle-Martin O, Malissen B, Abastado JP, Kourilsky P: The Vβ complementarity determining region 1 of a major histocompatibility complex (MHC) class I-restricted T cell receptor is involved in the recognition of peptide/MHC I and superantigen/MHC II complex. J Exp Med 1994;179: 1087–1097.
White J, Pullen A, Choi K, Marrack P, Kappler JW: Antigen recognition properties of mutant Vβ3+ T cell receptors are consistent with an immunoglobulin-like structure for the receptor. J Exp Med 1993;177:119–125.
Pontzer CH, Irwin, MJ, Gascoigne NRJ, Johnson HM: T-cell antigen receptor binding sites for the microbial superantigen staphylococcal enterotoxin A. Proc Natl Acad Sci USA 1992;89:7727–7731.
Vacchio MS, Kanagawa O, Tomonari K, Hodes RJ: Influence of T cell receptor Vα expression on Mlsa superantigen-specific T cell responses. J Exp Med 1992;175:1405–1408.
Smith HP, Le P, Woodland DL, Blackman, MA: T cell receptor α-chain influences reactivity to Mls-1 in Vβ8.1 transgenic mice. J Immunol 1992;149:887–896.
Woodland DL, Smith HP, Surman S, Le P, Wen R, Blackman MA: Major histocompatibility complex-specific recognition of Mls-1 is mediated by multiple elements of the T cell receptor. J Exp Med 1993;177:433–442.
Rock EP, Sibbald PR, Davis MM, Chien YH: CDR3 length in antigen-specific immune responses J Exp Med 1994;179:323–328.
Sprent J: T lymphocytes and the thymus; in Paul WE (ed): Fundamental Immunology, ed 3. New York, Raven Press, 1993, pp 80–81.
Torres BA, Griggs ND, Johnson HM: Bacterial and retroviral superantigens share a common binding region on class II MHC antigens. Nature 1993;364:152–154.
Jardetzky TS, Brown JH, Gorga JC, Stern LJ, Urban RG, Chi YI, Stauffacher C, Strominger JL, Wiley DC: Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature 1994;368:711–718.
Patten PA, Rock EP, Sonoda T, de Fazekas St, Groth B, Jorgensen JL, Davis MM: Transfer of putative complementarity-determining loops of T cell receptor V domains confers toxin reactivity but not peptide/MHC specificity. J Immunol 1993; 150:2281–2294.
Herman A, Croteau G, Sekaly RP, Kappler J, Marrack P: HLA-DR alleles differ in their ability to present staphylococcal enterotoxins to T cells. J Exp Med 1990;172:709–717.
Fraser JD: High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR Nature 1989;339:221–224.
Winslow GM, Scherer MT, Kappler JW, Marrack P: Detection and biochemical characterization of the mouse mammary tumour virus 7 superantigen (Mls-1a). Cell 1992;71:719–730.
Mollick JA, McMasters RL, Grossman D, Rich RR: Localization of a site on bacterial superantigens that determines T cell receptor β chain specificity. J Exp Med 1993;177: 283–293.
Choi Y, Marrack P, Kappler JW: Structural analysis of a mouse mammary tumor virus superantigen. J Exp Med 1992;175:847–852.
Bergdoll MS: Staphylococcal intoxication; in Riemann H, Bryan Fl (eds): Food-Borne Infections and Intoxications, ed 2, New York, Academic Press, 1979, pp 443–494.
Todd JK, Fishaut M, Kapral F, Welch T: Toxic shock syndrome associated with phage-group 1 staphylococci. Lancet 1978;ii:1116–1118.
Stevens DL, Tanner MH, Winship J: Group A streptococcal infections associated with toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 1989;321:1–7.
Imberti L, Sottini A, Bettinardi A, Puoti M, Primi D: Selective depletion in HIV infection of T cells that bear specific T cell receptor Vβ sequences. Science 1991;254:860–862.
Laurence J, Hodtsev AS, Posnett DN: Superantigen implicated in dependence of HIV-1 replication in T cells on TCR Vβ expression. Nature 1992;358:255–259.
Lafon M, Lafage M, Martinez-Arends A, Ramirez R, Vuillier F, Charron D, Lotteau V, Scott-Algara D: Evidence for a viral superantigen in humans. Nature 1992;358:507–510.
Cole BC, Ward JR, Jones RS, Cahill JF: Chronic proliferative arthritis of mice induced byMycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun 1971;4:344–355.
Cole BC, Balderas RA, Ahmed EA, Kono D, Theofilopoulos AN: Genomic composition and allelic polymorphisms influence Vβ usage by theMycoplasma arthritidis superantigen. J Immunol 1993;150:3291–3299.
Posnett DN: Do superantigens play a critical role in autoimmunity? Semin Immunol 1993;5:65–72.
Abe J, Kotzin BL, Jujo K, Melish ME: Selective expansion of T cells expressing TCR variable regions Vβ2 and Vβ8 in Kawasaki disease. Proc Natl Acad Sci USA 1992;8: 4066–4070.
Kotzin BL, Leung DY, Kappler J, Marrack P: Superantigens and their potential role in human disease. Adv Immunol 1993;54:99–166.
Sagle BL, Butel JS: Exogenous and endogenous mouse mammary tumor viruses: Replication and transformation; in Medina D, Kidwell W, Heppner G, Anderson E (eds): Cellular and Molecular Biology of Mammary Cancer. New York, Plenum Press, 1987, pp 275–306.
Choi Y, Kappler JW, Marrack P: A superantigen encoded in the open reading frame of the 3′ long terminal repeat of mouse mammary tumor virus. Nature 1991;350:203–207.
Acha-Orbea H, Shakhov AN, Scarpellino L, Kolb E, Muller V, Vessaz-Shaw A, Fuchs R, Blochlinger K, Rollini P, Billotte J, Sarafilder M, McDonald HR, Diggelman H: Clonal deletion of Vβ14-bearing T cells in mice transgenic for mammary tumor virus. Nature 1991;350:207–211.
Golovkina TV, Chervonsky A, Dudley JP, Ross SR: Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell 1992;69:637–645.
Acha-Orbea H, Held W, Waanders GA, Shakhov N, Scarpellino L, Lees RK, MacDonald HR: Exogenous and endogenous mouse mammary tumor virus superantigens. Immunol Rev 1993;131:5–25.
Lefebvre P, Berard DS, Cordingely MG, Hager GL: Two regions of the mouse mammary tumor virus long terminal repeat regulate the activity of its promoter in mammary cell lines. Mol Cell Biol 1991;11:2529–2537.
Kain SR, Platt EJ, Brown KS, Black N, Firestone GL: Disruptions in intracellular membrane trafficking and structure preclude the glucocorticoid-dependant maturation of mouse mammary tumor virus proteins in rat hepatoma cells. J Biol Chem 1992;267:8182–8132.
Pucillo CEM, Cepeda R, Hodes RJ: Expression of a MHC class II transgene determines both superantigenicity and susceptibility to mammary tumor virus infection. J Exp Med 1993;178:1441–1445.
Marrack P, Lo D, Brinster R, Palmiter R, Burkly L, Flavell KH, Kappler J: The effect of thymus environment on T cell development and tolerance. Cell 1988;53:627–634.
Waanders GA, Shakhov AN, Held W, Karapetian O, Acha-Orbea H, MacDonald HR: Peripheral T cell activation and deletion induced by transfer of lymphocyte subsets expressing endogenous or exogenous mouse mammary tumor virus. J Exp Med 1993;177:1359–1366.
Hodes RJ, Novick MB, Palmer LD, Knepper JE: Association of a Vβ2-specific superantigen with a tumorigenic milk-borne mouse mammary tumor virus. J Immunol 1993;150: 1422–1428.
Held W, Acha-Orbea H, MacDonald HR, Waanders GA: Superantigens and retroviral infection: Insight from mouse mammary tumor virus. Immunol Today 1994;15:184–190.
Bette M, Schäfer MKH, van Rooijen N, Weihe E, Fleischer B: Distribution and kinetics of superantigen-induced cytokine gene expression in mouse spleen. J Exp Med 1993;178:1531–1540.
Cardell S, Höidén I, Möller G: Manipulation of the superantigen-induced lymphokine response. Selective induction of interleukin-10 or interferon-γ synthesis in small resting CD4+ T cells. Eur J Immunol 1993;23:523–529.
Held W, Shakhov AN, Izui S, Waanders GA, Scarpellino L, MacDonald HR, Acha-Orbea H: Superantigen-reactive CD4+ cells are required to stimulate B cells after infection with mouse mammary tumor virus. J Exp Med 1993;177:359–366.
MacDonald HR, Baschieri S, Lees RK: Clonal expansion precedes anergy and death of Vβ8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol 1991;21:1963–1966.
Ignatowicz L, Kappler J, Marrack P: The effects of chronic infection with a superantigen-producing virus. J Exp Med 1992;175:917–923.
Beutner U, Kraus E, Kitamura D, rajewsky K, Huber BT: B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J Exp Med 1994;179: 1457–1466.
Gollob KJ, Palmer E: Aberrant induction of T cell tolerance in B cell suppressed mice. J Immunol 1993; 150:3705–3712.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pucillo, C.E.M., Palmer, L.D. & Hodes, R.J. Superantigenic characteristics of mouse mammary tumor viruses play a critical role in susceptibility to infection in mice. Immunol Res 14, 58–68 (1995). https://doi.org/10.1007/BF02918497
Issue Date:
DOI: https://doi.org/10.1007/BF02918497