Abstract
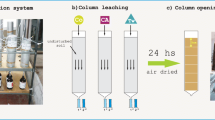
Recently, extensive research on soil and groundwater remediation has demonstrated surfactant (surface active agents) flushing to be a viable alternative for improving the efficiency of pump-and-treat remediation. Column tests were carried out to examine the effect of surfactant solution conditions on surfactant-enhanced remediation of soil columns contaminated by 1,2,4-trichlorobenzene (TCB). An Iowa Fruitfield soil was used for this study and sodium diphenyl oxide disulfonate (DOSL) that is an anionic surfactant was selected in this research. The conditioned parameters of the surfactant solution for the column tests were concentration, pH, temperature and flow rate. The test results revealed that an optimum condition was achieved for 4% (v/v) of concentration, pH 10, 20°C of temperature and 4 mL/min of flow rate, respectively. The removal of 98% of TCB was obtained with combination of optimal conditions of surfactant solution with NaCl. This was a marked improvement and removal efficiency increased by 4–17% compared to that with unadjusted (not optimum) conditions. The optimum range of these parameters may be useful for surfactant-based remediation in soil contaminated by TCB.
Similar content being viewed by others
References
Chen, L. and Knox, R.C., 1997, Using vertical circulation wells for partitioning tracer tests and remediation of DNAPLs. Ground Water Monitoring and Remediation, 17, 161–168.
Chang, M.-C., Huang, C.-R. and Shu, H.-Y., 2000, Effects of surfactants on extraction of phenanthrene in spiked sand. Chemosphere, 41, 1295–1300.
Gannon, O.K., Bibring, P., Raney, K., Ward, J.A., Wilson, D.J., Underwood, J.L. and Debelak, K.A., 1989, Soil clean up by insitu surfactant flushing. III. Laboratory results. Separation Science and Technology, 24, 1073–1094.
Gonzalez, J.M. and Ukrainczyk, L., 1996, Adsorption and desorption of nicosulfuron in soils. Environmenal Quality, 25, 1186–1192.
Jafvert, C.T. and Heath, J.K., 1991, Sediment and saturated soil associated reactions involving an anionic surfactant (Dodecylsulfate). 1. Precipitation and micelle formation. Environmental Science and Technology, 25, 1031–1038.
Joshi, M.M. and Lee, S., 1996, Optimization of surfactant-aided remediation of industrially contaminated soils. Energy Sources, 18, 291–3016.
Lee, D-H., 1997, The effect of surfactants in leaching hydrophobic organic compounds from sand. M.S. Thesis, Iowa State University, Ames, IA, 88 p.
Lee, D-H., 1999, Experimental investigation of the removal of hydrophobic organic compounds from two Iowa soils using food grade surfactants and recovering of used surfactants. Ph.D. Dissertation. Iowa State University, Ames, IA. 200 p.
Lee, D-H., Cody, R.D. and Hoyle, B.L., 2001, Laboratory evaluation of the use of surfactants for ground water remediation and the potential for recycling them. Groundwater Monitoring and Remediation, 21, 49–57.
Liu, M. and Roy, D., 1992, Washing of hydrophobic organic from contaminated sand with a surfactant. Minerals and Metallurgical Processing, 11, 206–208.
Mackay, D.M. and Cherry, J.A., 1989, Groundwater contamination: Pump-and-treat remediation. Environmental Science and Technology, 23, 630–636.
Martel, R. and Gelinas, P.J., 1996, Surfactant solutions developed for NAPL recovery in contaminated aquifers. Ground Water, 34, 143–154.
Pennell, K.D., Adinolfi, A.M., Abriola, L.M. and Diallo, M.S., 1997, Solubilization of dodecane, tetrachloroethylene, and 1,2-dichlorobenzene in micellar solutions of ethoxylated nonionic surfactants. Environmental Science and Technology, 31, 1382–1389.
Rosen, M.J., 1989, Surfactants and interfacial phenomena. John Wiley & Sons, New York. 1–68.
Rouse, J.D., Sabatini, D.A. and Harwell, J.H., 1993, Minimizing surfactant losses using twin-head anionic surfactants in subsurface remediation. Environmental Science and Technology, 27, 2072–2078.
Shiau, B.J., Sabatini, D.A. and Harwell, J.H., 1995, Properties of food grade (edible) surfactants affecting subsurface remediation of chlorinated solvents. Environmental Science and Technology, 29, 2929–2935.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, DH., Cody, R.D. Optimization of DOSL surfactant solution parameters on surfactant-enhanced remediation of soil columns contaminated by 1,2,4-trichlorobenzene. Geosci J 5, 281–286 (2001). https://doi.org/10.1007/BF02912698
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02912698