Summary
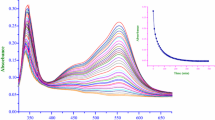
The kinetics of replacement of 4-cyanopyridine (4-CNpy) by CN− in [Fe(CN)5(4-CNpy)]3− have been studied in different concentrations of electrolyte. Plots of ln (k/kw)versus (γ−γw), where the subscript w refers to pure water and γ is the surface tension of the appropriate salt solution, gave a common straight line for all the electrolytes studied. This result seems to confirm theD character of the process studied and permits an estimate of the activation volume to be made from kinetic data.
Similar content being viewed by others
References
J. Burgess and E. Pelizzetti,Gazz. Chim. Ital.,118, 803 (1988);Prog React. Kinet., in press.
G. C. Pedrosa, J. A. Salas and M. Katz,J. Coord. Chem.,12, 145 (1983).
G. C. Pedrosa, N. Hernández, N. E. Katz and M. Katz,J. Chem. Soc., Dalton Trans., 2297 (1980).
J. Benko, O. Vollarova, O. Grancicova and V. Holba,J. Coord. Chem.,14, 175 (1985).
M. A. Blesa, E. Borghi and R. Fernández-Prini,J. Chem. Soc., Faraday Trans. 1,81, 3021 (1985).
M. J. Blandamer, J. Burgess and R. I. Haines,J. Chem. Soc., Dalton Trans., 244 (1978).
M. L. Moyá, A. Barrios, M. M. Graciani, R. Jiménez, E. Muñoz, F. Sánchez and J. Burgess,Transition Met. Chem.,16, 165 (1991).
M. J. Blandamer, J. Burgess, K. Morcom and R. Sherry,Transition Met. Chem.,8, 354 (1982).
A. P. Szecsy, S. S. Miller and A. Haim,Inorg. Chim. Acta,28, 189 (1978).
E. A. Abu-Gharib, R. Ben Ali, M. J. Blandamer and J. Burgess,Transition Met. Chem.,12, 371 (1989).
See,e.g., M. H. M. Abou-El-Wafa, M. G. Burnett and J. F. McCullagh,J. Chem. Soc., Dalton Trans., 2083 (1986); J. Burgess,Mech. Inorg. Organometal. React.,5, 191 (1988);6, 210 (1989); and references therein.
H. Elias, H-T Macholdt, K. J. Wannowius, M. J. Blandamer, J. Burgess and B. Clark,Inorg. Chem.,25, 3048 (1986).
T. R. Sullivan, D. R. Stranks, J. Burgess and R. I. Haines,J. Chem. Soc., Dalton Trans., 1460 (1977).
J. P. Postma, H. J. C. Berendsen and J. R. Haan,Faraday Symp. Chem. Soc.,17, 55 (1982).
H. Reiss,Actv. Chem. Phys.,IX, 1 (1965).
International Critical Tables, vol. IV, McGraw-Hill Book Company Inc., New York-London, 1928.
R. Ben Ali, M. J. Blandamer, J. Burgess, P. Guardado and F. Sánchez,Inorg. Chim. Acta,131, 59 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barrios, A., Graciani, M.d.M., Jiménez, R. et al. Salt effects on the kinetics of dissociation of the pentacyano-4-cyanopyridineferrate(II) anion. Transition Met. Chem. 17, 231–234 (1992). https://doi.org/10.1007/BF02910844
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02910844