Summary
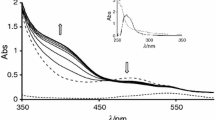
The proton dissociation pKa data for RuII(hedta)(LH) and Ru II2 (ttha)(LH)2 complexes were measured by the spectrophotometric titration mid-point method using the difference in the MLCT spectra for the protonated and deprotonated complexes with L=2-methylpyrazine (2-Mepz) and 4,4′-bipyridine (4,4′-bpy). The pK ′a s were as follows at 22° C, μ=0.10 (NaCl): Ru(hedta)(2-MepzH), 3.45; Ru2(ttha)(2-MepzH)2, 3.25; Ru(hedta)(4,4′-bpyH), 4.5; Ru2(ttha)(4,4′-bpyH)2, 4.51. The two series (2-Mepzversus 4,4′-bpy) lead to a divergent prediction of the ability of RuII (polyaminopolycarboxylates) to serve as π-donor metal centers in comparison with RuII(NH3) 2+5 . The results with 4,4′-bpy ligation, where the site of protonation is more isolated from electrostatic effects of the ruthenium center, appear to give the more correct interpretation, consistent with their capacity to make good π-complexes with olefins and pyrimidine nucleobases, that RuII(polyaminopolycarboxylates) are better π-donor metal centers than Ru(NH3) 2+5 ·ΔpK*, the difference between ground state and excited state pK ′a s, are used to establish the π-donating order of RuII(polyaminopolycarboxylates) as compared to Ru(NH3) 2+5 and Ru(CN) 3−5 . The π-donor effect of the ruthenium(II) center in Ru II2 (ttha)(1,3-butadiene) 2−2 is observed to influence the ligand electronically up to five bonds away from the site of metallation.
Similar content being viewed by others
References
P. Ford, D. P. Rudd, R. G. Gaunder and H. Taube,J. Am. Chem. Soc.,90, 1187 (1968).
C. R. Johnson and R. E. Shepherd,Inorg. Chem.,22, 1117 (1983).
H. E. Toma and E. Stadler,Inorg. Chem.,24, 3085 (1985).
M. G. Elliott, S. Zhang and R. E. Shepherd,Inorg. Chem.,28, 3036 (1989).
S. Zhang and R. E. Shepherd,Inorg. Chem.,27, 4712 (1988).
S. Zhang, L. A. Holl and R. E. Shepherd,Inorg. Chem.,29, 1012 (1990).
S. Zhang and R. E. Shepherd,Inorg. Chim. Acta,163, 237 (1989).
S. Zhang, Ph.D. Thesis, University of Pittsburgh (USA), 1991.
S. Zhang and R. E. Shepherd, submitted toInorg. Chim. Acta, 1991. (b) S. Zhang and R. E. Shepherd, submitted toInorg. Chem., (1991).
S. Zhang and R. E. Shepherd,Transition Met. Chem., in Press (1991).
R. E. Shepherd, A. Proctor, W. W. Henderson and T. K. Myser,Inorg. Chem.,26, 2440 (1987).
S. Zhang and R. E. Shepherd,Inorg. Chim. Acta, in press (1991).
R. E. Shepherd, S. Zhang, P. Dowd, G. Choi, B. Wilk andInorg. Chem. Acta,174, 149 (1990).
M. G. Elliott and R. E. Shepherd,Inorg. Chem.,27, 3332 (1988).
A. A. Diamantis and J. V. Dubrawski,Inorg. Chem.,20, 1142 (1981).
T. Matsubara and C. Creutz,Inorg. Chem.,18, 1956 (1979). (b) T. Matsubara and C. Creutz,J. Am. Chem. Soc.,100, 6255 (1978).
Characterization is described in the experimental section. This work was previously reported; S. Zhang, T. K. Myser and R. E. Shepherd, 10th Central Regional American Chemical Society Meeting, Columbus, Ohio, June 25, 1987.
W. R. McWinnie and J. D. Miller,Adv. Inorg. Chem. Radiochem,12, 135 (1969). (b) D. N. Lawson and G. Wilkinson,J. Chem. Soc. 1900 (1965). (c) L. L. Merritt and E. D. Schroeder,Acta Cryst. 9, 801 (1956). (d) R. J. W. LeFevre,J. Chem. Soc. 1773 (1963). (e) C. W. M. Cureton, R. F. A. Guinman and A. I. J. Vogel,J. Chem. Soc., 1188 (1962).
G. Jackson and G. Porter,Proc. R. Soc. London, Ser. A,260, 13 (1961).
J. Sen and H. Taube,Acta Chem. Scand. Ser. A,A33, 125 (1979).
D. K. Lavallee and E. B. Fleischer,J. Am. Chem. Soc.,94, 2583 (1972).
M. S. Ram and A. Haim,Inorg. Chem.,30, 1319 (1991).
C. R. Johnson and R. E. Shepherd,Inorg. Chem.,22, 2439 (1983).
S. Siddiqui, W. W. Henderson and R. E. Shepherd,Inorg. Chem.,26, 3101 (1987).
S. Zhang and R. E. Shepherd,Inorg. Chim. Acta, in press (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, S., Shepherd, R.E. Protonation of coordinated 2-methylpyrazine and 4,4′-bipyridine as a probe of π-donor potential of ruthenium(II) polyaminopolycarboxylate complexes. Transition Met. Chem. 17, 199–203 (1992). https://doi.org/10.1007/BF02910836
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02910836