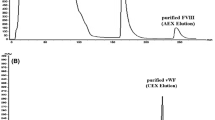
Summary
In vitro andin vivo characteristics of cryoprecipitates and three commercial factor VIII concentrates (Kryobulin, Hemofil and Koate) were comparatively studied. Factor VIII:C/VIII:Ag ratio was very low in all commercial concentrates without differences among them. Conversely, the decrease of factor VIIIR:WFRCof was proportional to the degree of purity. Factor VIII:C/VIIIR:WFRCof ratio was shown to be a reliable index of factor VIII complex denaturation. Crossed electroimmunoassay showed a faster migration of factor VIIIR:Ag only in commercial concentrates. Using a two-compartmental open model that accounts for endogenous synthesis of factor VIII:C, single-dose kinetics of factor VIII:C were studied in 23 patients with classic hemophilia. Good agreement between measured and fitted values of factor VIII:C plasma concentration was observed. β half-life was shorter in high purity concentrates and longer in intermediate purity concentrates and cryoprecipitates.
Similar content being viewed by others
References
Allain J. P., Verroust F., Soulier J. P.:In vitro andin vivo characterization of factor VIII preparations—Vox Sang (Basel)38, 68, 1980.
Bennett B., Ratnoff O. D.: Studies on the response of patients with classic hemophilia to transfusion with concentrates of antihemophilic factor: a difference in the half-life of anti-hemophilic factor as measured by procoagulant and immunologic techniques—J. clin. Invest.51, 7593, 1972.
Biggs R., Denson K. W. E.: The fate of prothrombin and factors VIII, IX and X transfused to patients deficient of these factors—Brit. J. Haematol.9, 532, 1963.
Blatt P. M., Brinkhous K. M., Culp H. R., Krauss J. S., Roberts H. R.: Antihemophilic factor concentrate therapy in von Willebrand’s disease. Dissociation of bleeding-time factor and ristocetin cofactor activities—J. Amer. med. Ass.236, 1770, 1976.
Clarke M. G. M., Freeman T.: A quantitative immunoelectrophoresis method (Laurell’s electrophoresis). In:Peeters H. H. (Ed.): Protides of biological fluids. Elsevier, Amsterdam, 1967; p. 503.
Clauss A.: Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens— Acta haematol. (Basel)17, 237, 1957.
Finney D. J.: Statistical methods in biological assay. Griffin C. and Co., London, 1978.
Gibaldi M., Perrier D.: Pharmacokinetics. Marcel Dekker, New York, 1975; p. 281.
Green D., Potter E. V.: Failure of AHF concentrate to control bleeding in von Willebrand’s disease—Amer. J. Med.60, 357, 1976.
Greenblatt D. J., Koch-Weser J.: Clinical pharmacokinetics—New Engl. J. Med.293, 702, 1975.
Hermens W. T.: Dose calculation of human factor-VIII and factor-IX concentrates for infusion therapy. In:Brinkhous K. M., Hemker H. C. (Eds): Handbook of hemophilia. Excerpta Medica, Amsterdam, 1975; p. 569.
Ingram G. I. C.: Blood coagulation factor VIII, genetics, physiological control, and bioassay— Advanc. clin. Chem.21, 189, 1965.
Jakab T., Pflugshaupt R., Furlan M., Beck E. A.: Variable degradation of factor VIII-related protein in lyophilized concentrates of antihaemophilic factor—Vox Sang. (Basel)35, 36, 1978.
Laurell C.-B.: Quantitative estimation of proteins by electrophoresis in agarose gel-containing antibodies—Ann. Biochem.15, 45, 1966.
Lombardi R., Mannucci P. M., Seghatchian M. J., Garcia V. V., Coppola R.: Alterations of factor VIII von Willebrand factor in clinical conditions associated with an increase in its plasma concentration—Brit. J. Haematol.49, 61, 1981.
Lowry O. H., Rosenbrough N. J., Farr A. L., Randall R. J.: Protein measurement with the Folin-phenol reagent—J. biol. Chem.193, 265, 1951.
Mancini G., Carbonara A. O., Heremans J. F.: Immunochemical quantitation of antigens by single radial immunodiffusion—Immunochemistry2, 235, 1965.
McCue M. J., Brossoit A. D., Marmer D. J., Head D. R.: Von Willebrand factor (VIII:VWF) in lyophilized factor VIII concentrates—Amer. J. Haematol.9, 39, 1980.
Montgomery R. R., Johnson J.: Specific factor VIII-related antigen fragmentation: anin vivo andin vitro phenomenon—Blood60, 930, 1982.
Nilsson I. M., Hedner U.: Characteristics of various factor VIII concentrates used in treatment of haemophilia A—Brit. J. Haematol.37, 543, 1977.
Nilsson I. M., Holmberg L., Stenberg P., Henriksson P.: Characteristics of the factor VIII protein and factor XIII in various factor VIII concentrates—Scand. J. Haematol.24, 340, 1980.
Over J., Sixma J. J., Doucet-de-Bruine M. H. M., Trieschnigg A. M. G., Vlooswijk R. A. A., Beeser-Visser N. H., Bouma B. N.: Survival of 125-iodine-labeled factor VIII in normals and patients with classic hemophilia—J. clin. Invest.62, 223, 1978.
Pool J. G., Robinson J.: Observations on plasma banking and transfusion procedures for haemophilic patients using a quantitative assay for antihaemophilic globulin (AHG)—Brit. J. Haematol.5, 24, 1959.
Pool J. G., Shannon A. E.: Production of high-potency concentrates of anti-haemophilic globulin in a closed-bag system—New Engl. J. Med.273, 1443, 1965.
Prowse C. V., Griffin B., Pepper D. S., Dickson A. J., McQuillan T. A., Dickson I. H., Foster P. R.: Changes in factor VIII complex activities during the production of a clinical intermediate purity factor VIII concentrate—Thrombos. Haemost.46, 597, 1981.
Shapiro G. A., Andresen J. C., Pizzo S. V., McKee P. A.: The subunit structure of normal and haemophilic factor VIII—J. clin. Invest.52, 2198, 1973.
Smith K. J., Thompson A. R.: Labeled factor IX kinetics in patients with hemophilia B—Blood58, 625, 1981.
van Gastel C., Sixma J. J., Borst Eilers E., Leautaud M., Moes M., van der Plas M., Bouma B. N., Sybesma J. P.: Preparation and infusion of cryoprecipitate from exercised donors—Brit. J. Haematol.25, 461, 1973.
Verstraete M., Vermylen J.: Laboratory and clinical evaluation of concentrates for treatment of haemophilia—Acta clin. belg.30, 437, 1975.
Wagner J. G.: Fundamentals of clinical pharmacokinetics. Drug Intelligence Publ., Hamilton, 1975; p. 81.
Weinstein M., Deykin D.: Comparison of factor VIII-related von Willebrand factor proteins prepared from human cryoprecipitate and factor VIII concentrate—Blood53, 1095, 1979.
Weiss H. J., Hoyer L. J., Rickles F. R., Varma A., Rogers J.: Quantitative assay of a plasma factor deficient in von Willebrand disease that is necessary for platelet aggregation—J. clin. Invest.53, 2708, 1973.
Zimmerman T. S., Roberts J., Edington T. S.: Factor VIII-related antigen: multiple molecular forms in human plasma—Proc. nat. Acad. Sci. (Wash.)72, 5121, 1975.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morfini, M., Longo, G., Matucci, M. et al. Cryoprecipitate and factor VIII commercial concentrates:In vitro characteristics andin vivo compartmental analysis. La Ricerca Clin. Lab. 14, 681–691 (1984). https://doi.org/10.1007/BF02906309
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02906309