Summary
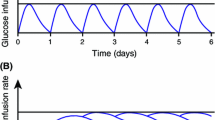
The aim of this study was to evaluate the metabolic effects of a new synthetic ACTH analogue (ACTH 1–17) in insulin-dependent diabetic subjects. ACTH 1–17 (100 μg, intramuscular injection) was administered at 0700–0730 every second day for 20 days. Changes in insulin dosage were carried out to maintain the same metabolic control during the period of the study. Before and after treatment diurnal plasma glucose profiles were superimposable and insulin requirement increased only in 16 out of 19 patients (mean: 6.7±2 U/die; range: 2–22 U/die). No changes were observed in diurnal profiles of blood alanine, glycerol, total ketone bodies and plasma NEFA, C-peptide, glucagon. The physiological blood lactate and pyruvate peaks following the evening meal were initially absent and could be detected after treatment. From our data it is not clear whether the more physiological pattern of blood lactate and pyruvate is caused by the modest increase in insulin dosage or is a specific effect of the treatment.
Similar content being viewed by others
References
Angeli A., Carandente F., Halberg F.: Temporal aspects of glucocorticoid action and clinical implications—La Ricerca Clin. Lab.13, 203, 1983.
Angeli A., Paccotti P., Orlandi F., Gaidano G., Ceresa F.: Differential patterns of plasma cortisol and aldosterone following stimulation with increasing doses of the synthetic analogue (B-Ala1, Lys17) ACTH 1–17-4-amino-N-butylamide—Hormone metab. Res.13, 24, 1981.
Baker L., Kaye R., Root A. W.: The early partial remission of juvenile diabetes mellitus. The role of insulin and growth hormone—J. Pediatr.71, 825, 1967.
Bergmeyer H. V.: Methods of enzymatic analysis. Academic Press, New York-London, 1974.
Bottazzo G. F., Pujol-Borrel R., Doniach D.: Humoral and cellular immunity in diabetes mellitus—Clin. Immunol. Allergy1, 63, 1981.
Cahill G.: Action of adrenal cortical steroids on carbohydrate metabolism. In:Christy N. (Ed.): The human adrenal cortex. Harper Row, New York, 1971; p. 205.
Conn J. W., Fajans S. S.: Influence of adrenal cortical steroids on carbohydrate metabolism in man—Metabolism5, 114, 1956.
Doar J. W. H., Cramp D. G., Maw D. S. J., Seed M., Wynn V.: Blood pyruvate and lactate levels during oral and intravenous glucose tolerance tests in diabetes mellitus—Clin. Sci.39, 259, 1970.
Duncombe W. C.: The colorimetric microdetermination of NEFA in plasma—Clin. chim. Acta9, 122, 1964.
Felig P., Wahren J., Hendler R.: Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man—Diabetes24, 468, 1975.
Lebovitz H. E., Bryant K., Frohman L. A.: Acute effects of corticotropin and related peptides on carbohydrate and lipid metabolism—Ann. N.Y. Acad. Sci.131, 274, 1965.
Madsbad S., Faber O. N., Binde C., Alberti K. G. M. M., Lloyd B.: Diurnal profiles of intermediary metabolites in insulin-dependent diabetes and their relationship to different degrees of residual B—cell function—Acta diabetol. lat.18, 115, 1981.
Mc Kiddie M. T., Jasani M. K., Buchanan K. D., Boyle J. A., Buchanan W. W.: The relationship between glucose tolerance, plasma insulin and corticosteroid therapy in patients with rheumatoid arthritis—Metabolism17, 730, 1968.
Nosadini R., Del Prato S., Tiengo A., Valerio A., Muggeo M., Opocher G., Mantero F., Dunes E., Marescotti C., Mallo F., Belloni F.: Insulin resistance in Cushing’s syndrome—J. clin. Endocrinol.57, 529, 1983.
Olefsky J., Kimmerling G.: Effects of glucocorticoid on carbohydrate metabolism—Amer. J. med. Sci.271, 202, 1976.
Reinberg A., Guillemant S., Ghata N. J., Guillemant J., Touitou Y., Dupont W., Lagoguey M., Bourgeois Ph., Brière L., Fraboulet G., Guillet P.: Clinical chronopharmacology of ACTH 1–17. Effects on plasma cortisol and urinary 17-hydroxycorticosteroids—Chronobiologia7, 513–523, 1980.
Rizza R. A., Mandarino L. J., Gerich J. E.: Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action—J. clin. Endocrinol.54, 131, 1982.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prando, R., Buzzo, P., Cheli, V. et al. ACTH 1–17 effects in insulin dependent diabetes mellitus. La Ricerca Clin. Lab. 14, 181–188 (1984). https://doi.org/10.1007/BF02904971
Issue Date:
DOI: https://doi.org/10.1007/BF02904971