Abstract
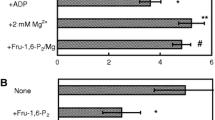
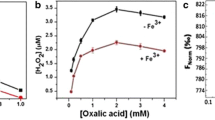
It has been investigated whether oxalomalate (OMA) the competitive inhibitor of cytosolic aconitase might extend its effect also on IRP-1, an iron regulatory protein, probably present in a commercial preparation of purified aconitase. The results showed that IRP-1 is contained in the purified preparation and was strongly inhibited during the incubation with OMA. The effect produced by OMA on the bi-functional protein IRP-1/aconitase was discussed.
Riassunto
È stato indagato l’effetto dell’OMA, ben noto inibitore competitivo della forma citosolica di aconitasi, sulla eventuale presenza di IRP-1 in una preparazione commerciale di aconitasi purificata. I risultati hanno dimostrato che IRP-1 è presente nella preparazione purificata e viene fortemente inibita nel corso di una breve incubazione con OMA 5 mM, a temperatura ambiente. Viene messa in evidenza l’inibizione prodotta da OMA su entrambe le forme della proteina bifunzionale IRP-1/Aconitasi.
Similar content being viewed by others
References
Eisenstein R. S., Blemings K. P., 1998.Iron regulatory Proteins, iron responsive elements and iron homeostasis. J. Nutr., 128: 2295–2298.
Festa M., Colonna A., Pietropaolo C., Ruffo A., 1999.Regulation of iron metabolism by oxalomalate. Rend. Fis. Acc. Lincei, s. 9, v. 10: 21–24.
Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D., 1992.Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulphur cluster results in high-affinity RNA binding. Proc. Natl. Acad. Sci. USA, 89: 11735–11739.
Hentze M. W., Kühn L. C., 1996.Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc. Natl. Acad. Sci. USA, 93: 8175–8182.
Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert, H., 1983.The role of iron in the activation-inactivation of aconitase. J. Biol. Chem., 258: 11098–11105.
Klausner R. D., Rouault T. A., Harford J. B., 1993.Regulating the fate of mRNA: the control of cellular iron metabolism. Cell, 72: 19–28.
Melefors Ö., Hentze M. W., 1993.Iron regulatory factor — the conductor of cellular iron regulation. Blood Reviews, 7: 251–258.
Philpott C. C., Haile D., Rouault T. A., Klausner R. D., 1993.Modification of a free Fe-S cluster cysteine residue in the active iron-responsive element-binding protein prevents RNA binding. J. Biol. Chem., 268: 17655–17658.
Philpott C. C., Klausner R. D., Rouault T. A., 1994.The bifunctional iron-responsive element binding protein/cytosolic aconitase: The role of active-site residues in ligand binding and regulation. Proc. Natl. Acad. Sci. USA, 91: 7321–7325.
Rouault T. A., Klausner R. D., 1996.Iron-sulfur clusters as biosensors of oxidants and iron. TIBS, 21: 174–177.
Author information
Authors and Affiliations
Additional information
Nella seduta del 10 marzo 2000.
Rights and permissions
About this article
Cite this article
Festa, M., Ruffo, A. Oxalomalate: an inhibitor for two functions. Rend. Fis. Acc. Lincei 11, 111–114 (2000). https://doi.org/10.1007/BF02904377
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02904377