Abstract
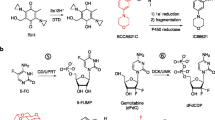
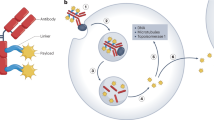
At present, chemotherapy is not very effective against common solid cancers especially once they have metastasised. However, laboratory experiments and studies on dose intensification in humans have indicated that some anti-cancer agents might be curative but only if the dose given was very much higher than that presently obtainable clinically. Prodrugs, activated by enzymes expressed at raised level in tumors, can deliver at least 50-fold the normal dose and can cure animals with tumors normally resistant to chemotherapy. This approach has not yet proved to be practicable clinically because of the rarity of human tumors expressing a high level of an activating enzyme. However, new therapies have been proposed overcome this limitation of prodrug therapy. Enzymes that activate prodrugs can be directed to human tumor xenografts by conjugating them to tumor associated antibodies. After allowing for the conjugate to clear from the blood a prodrug is administered which is normally inert but which is activated by the enzyme delivered to the tumor. This procedure is referred to as ADEPT (antibody-directed enzyme prodrug therapy). Early clinical trials are promising and indicate that ADEPT may become an effective treatment for all solid cancers for which tumor associated or tumor specific antibodies are known. Tumors have also been targeted with the genes encoding for a prodrug activating enzymes. This approach has been called genedirected enzyme prodrug therapy (GDEPT) or VDEPT (virus-directed enzyme prodrug therapy) and has shown good results in animal models. These new therapies may finally realise the potential of prodrugs in cancer chemotherapy.
Similar content being viewed by others
References
Knox RJ, Friedlos F, Marchbank T and Roberts JJ: Bioactivation of CB 1954: reaction of the active 4-hydroxylamino derivative with thioesters to form the ultimate DNA- DNA interstrand crosslinking species. Biochem Pharmacol 42:1691–1697, 1991.
Knox RJ, Friedlos F and Boland MP: The bioactivation of CB 1954 and its use as a prodrug in antibody-directed enzyme prodrug therapy (ADEPT). Cancer Metastasis Rev 12:195–212, 1993.
Roberts JJ, Friedlos F and Knox RJ: CB 1954 (2,4-dinitro-5- aziridinyl benzamide) becomes a DNA interstrand crosslinking agent in Walker tumor cells. Biochem Biophys Res Commun 140:1073–1078, 1986.
Cobb LM, Connors TA, Elson LA, et al: 2,4-dinitro-5-ethyl- eneiminobenzamide (CB 1954): a potent and selective inhibitor of the growth of the Walker carcinoma 256. Biochem Pharmacol 18:1519–1527, 1969.
Connors TA and Melzack DH: Studies on the mechanism of action of 5-aziridinyl-2,4-dinitrobenzamide (CB 1954), a selective inhibitor of the Walker tumor. Int J Cancer 7:86–92, 1971.
Connors TA and Whisson ME: Cure of mice bearing advanced plasma cell tumors with aniline mustard: the relationship between glucuronidase activity and tumor sensitivity. Nature 210:866–867, 1966.
Connors TA, Jeney A, Warwick GP and Whisson ME: Some factors influencing the sensitivity of tumors to nitrogen mustards. In: Isotopes in Experimental Pharmacology (Ed. Roth LJ), pp. 433–438. University of Chicago Press, Chicago and London, 1965.
Carl PL, Chakravarty PK and Katzenellenbogen JA: A novel connector linkage applicable in prodrug design. J Med Chem 24:479–480, 1981.
Manson MM, Legg RF, Watson JV, et al: An examination of the relative resistances to aflatoxin B1 and susceptibilities to γ-glu- tamyl para-phenylene diamine mustard of γ-glutamyl transferase negative and positive cell lines. Carcinogenesis 2:661- 670, 1981.
Boland MP, Knox RJ and Roberts JJ: The differences in kinetics of rat and human DT diaphorase result in a differential sensitivity of derived cell lines to CB 1954 (5-(aziridin-1- yl)-2,4-dinitrobenzamide). Biochem Pharmacol 41:867–875, 1991.
Young CW, Yagoda A, Bittar ES, et al: Therapeutic trial of aniline mustard in patients with advanced cancer. Comparison of therapeutic response with cytochemical assessment of tumor cell β-glucuronidase activity. Cancer 38:1887–1895, 1976.
Ehrlich P: The collected papers of P. Ehrlich. Pergammon Press, London, 1960.
Shockley TR, Lin K, Nagy JA, et al: Spatial distribution of tumor-specific monoclonal antibodies in human melanoma xenografts. Cancer Res 52:367–376, 1992.
Wawrzynczak EJ: Systemic immunotoxin therapy of cancer: advances and prospects. Br J Cancer 64:624–630, 1991.
Pimm MV: Drug-momoclonal antibody conjugates for cancer therapy: potential and limitations. CRC Crit Rev Ther Drug Carrier Syst 5:189–227, 1988.
Bagshawe KD, Springer CJ, Searle F, et al: A cytotoxic agent can be generated selectively at cancer sites. Br J Cancer 58:700–703, 1988.
Bagshawe KD: Antibody directed enzymes revive anti-cancer prodrugs concept. Br J Cancer 56:531–532, 1987.
Bagshawe KD: ADEPT and related concepts. Cell Biophys 25:83–91, 1994.
Bagshawe KD: Antibody-directed enzyme prodrug therapy - a review. Drug Development Research 34:220–230, 1995.
Knox RJ and Connors TA: Antibody-directed enzyme prodrug therapy. Clin Immunother 3:136–153, 1995.
Melton RG, Knox RJ and Connors TA: Antibody-directed enzyme prodrug therapy (ADEPT). Drugs Fut 21:167–181, 1996.
Melton RG and Sherwood RF: Antibody-enzyme conjugates for cancer therapy. J Natl Cancer Inst 88:153–165, 1996.
Stanislawski M, Rousseau V, Goavec M and Ito H: Immunotoxins containing glucose oxidase and lactoperoxidase with tumoricidal properties: in vitro killing effectiveness in a mouse plasmacytoma cell model. Cancer Res 49:5497- 5504, 1989.
Bagshawe KD, Sharma SK, Springer CJ, et al: Antibody directed enzyme prodrug therapy (ADEPT): clinical report. Dis Markers 9:233–238, 1991.
Bagshawe KD, Sharma SK, Springer CJ and Antoniw P: Antibody directed enzyme prodrug therapy: a pilot-scale clinical trial. Tumor Targeting 1:17–29, 1995.
Springer CJ, Poon GK, Sharma SK and Bagshawe KD: Identification of prodrug, active drug, and metabolites in an ADEPT clinical study. Cell Biophys 22:9–26, 1993.
Dowell RI, Springer CJ, Davies DH, et al: New mustard pro- drugs for antibody-directed enzyme prodrug therapy: alterna- tives to the amide link. J Med Chem 39:1100–1105, 1996.
Partridge LJ: Production of catalytic antibodies using combinatorial libraries. Biochem Soc Trans 21:1096–1098, 1993.
Chester KA, Begent RH, Robson L, et al: Phage libraries for generation of clinically useful antibodies. Lancet 343:455- 456, 1994.
Goshorn SC, Svensson HP, Kerr DE, et al: Genetic construc- tion, expression, and characterization of a single chain anticarcinoma antibody fused to β-lactamase. Cancer Res 53:2123–2127, 1993.
Bosslet K, Czech J, Seemann G, et al: Fusion protein mediated prodrug activation (FMPA) in vivo. Cell Biophys 25:51–63, 1994.
Wentworth P, Datta A, Blakey D, et al: Toward antibody- directed “abzyme” prodrug therapy, ADAPT: carbamate prodrug activation by a catalytic antibody and its in vitro application to human tumor cell killing. Proc Natl Acad Sci U S A 93:799–803, 1996.
Oldfield EH, Ram Z, Culver KW, et al: Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther 4:39–69, 1993.
Hart IR and Vile RG: Targeted gene therapy. Br Med Bull 51:647–655, 1995.
Consalvo M, Mullen CA, Modesti A, et al: 5-Fluorocytosine- induced eradication of murine adenocarcinomas engineered to express the cytosine deaminase suicide gene requires host immune competence and leaves an efficient memory. J Immunol 154:5302–5312, 1995.
Freeman SM, Whartenby KA, Freeman JL, et al: In situ use of suicide genes for cancer therapy. Semin Oncol 23:31–45, 1996.
Mullen CA, Coale MM, Lowe R and Blaese RM: Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res 54:1503–1506, 1994.
Mullen CA: Metabolic suicide genes in gene therapy. Pharmacol Ther 63:199–207, 1994.
Robinson DF and Maxwell IH: Suppression of single and double nonsense mutations introduced into the diphtheria toxin A- chain gene: a potential binary system for toxin gene therapy. Hum Gene Ther 6:137–143, 1995.
Cook DR, Maxwell IH, Glode LM, et al: Gene therapy for B- cell lymphoma in a SCID mouse model using an immunoglobulin-regulated diphtheria toxin gene delivered by a novel adenovirus-polylysine conjugate. Cancer Biother 9:131–141, 1994.
Maxwell IH, Glode LM and Maxwell F: Expression of diphtheria toxin A-chain in mature B-cells: a potential approach to therapy of B-lymphoid malignancy. Leuk Lymphoma 7:457- 462, 1992.
Maxwell IH, Glode LM and Maxwell F: Expression of the diphtheria toxin A-chain coding sequence under the control of promoters and enhancers from immunoglobulin genes as a means of directing toxicity to B-lymphoid cells. Cancer Res 51:4299–4304, 1991.
Mendelsohn ML: The growth fraction: a new concept applied to tumors. Science 132:1496, 1960.
Miller N and Vile R: Targeted vectors for gene therapy. FASEB J. 9:190–199, 1995.
Huber BE, Richards CA and Krenitsky TA: Retroviral-mediat- ed gene therapy for the treatment of hepatocellular carcinoma: an innovative approach for cancer therapy. Proc Natl Acad Sci U S A 88:8039–8043, 1991.
Borrelli E, Heyman R, Hsi M and Evans RM: Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci USA 85:7572–7576, 1988.
Moolten FS, Wells JM and Mroz PJ: Multiple transduction as a means of preserving ganciclovir chemosensitivity in sarcoma cells carrying retrovirally transduced herpes thymidine kinase genes. Cancer Lett 64:257–263, 1992.
Abe A, Takeo T, Emi N, et al: Transduction of a drug-sensitive toxic gene into human leukemia cell lines with a novel retroviral vector. Proc Soc Exp Biol Med 203:354–359, 1993.
Barba D, Hardin J, Ray J and Gage FH: Thymidine kinasemediated killing of rat brain tumors. J Neurosurg 79:729–735, 1993.
Vile RG and Hart IR: Use of tissue specific expression of the herpes simplex virus thymidine kinase gene to inhibit growth of established murine melanomas following direct intratu- moral injection of DNA. Cancer Res 53:3860–3864, 1993.
Chen SH, Shine HD, Goodman JC, et al: Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci USA 91:3054–3057, 1994.
Smythe WR, Hwang HC, Amin KM, et al: Use of recombinant adenovirus to transfer the herpes simplex virus thymidine kinase (HSVtk) gene to thoracic neoplasms: an effective in vitro drug sensitization system. Cancer Res 54:2055–2059, 1994.
Tanaka T, Kanai F, Okabe S, et al: Adenovirus-mediated pro-drug gene therapy for carcinoembryonic antigen-producing human gastric carcinoma cells in vitro. Cancer Res 56:1341–1345, 1996.
Tong XW Block A, Chen SH, et al: In vivo gene therapy of ovarian cancer by adenovirus-mediated thymidine kinase gene transduction and ganciclovir administration. Gynecol Oncol 61:175–179, 1996.
Tong XW, Block A, Chen SH, et al: Adenovirus-mediated thymidine kinase gene transduction in human epithelial ovarian cancer cell lines followed by exposure to ganciclovir. Anticancer Res 16:1611–1617, 1996.
Culver KW, Ram Z, Wallbridge S, et al: In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science 256:1550–1552, 1992.
Ram Z, Culver KW, Walbridge S, et al: Toxicity studies of retroviral-mediated gene transfer for the treatment of brain tumors. J Neurosurg 79:400–407, 1993.
Caruso M, Panis Y, Gagandeep S, Houssin D, Salzmann JL and Klatzmann D: Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci USA 90:7024–7028, 1993.
Manome Y, Abe M, Hagen MF, et al: Enhancer sequences of the DF3 gene regulate expression of the herpes simplex virus thymidine kinase gene and confer sensitivity of human breast cancer cells to ganciclovir. Cancer Res 54:5408–5413, 1994.
O’Malley B, Jr., Chen SH, Schwartz MR and Woo SL: Adenovirus-mediated gene therapy for human head and neck squamous cell cancer in a nude mouse model. Cancer Res 55:1080–1085, 1995.
Osaki T, Tanio Y, Tachibana I, et al: Gene therapy for carci-noembryonic antigen-producing human lung cancer cells by cell type-specific expression of herpes simplex virus thymidine kinase gene. Cancer Res 54:5258–5261, 1994.
Vile RG, Nelson JA, Castleden S, et al: Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res 54:6228–6234, 1994.
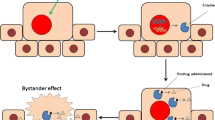
Freeman SM, Abboud CN, Whartenby KA, et al: The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res 53:5274–5283, 1993.
Bi WL, Parysek LM, Warnick R and Stambrook PJ: In vitro evidence that metabolic cooperation is responsible for the bystander effect observed with HSV tk retroviral gene therapy. Hum Gene Ther 4:725–731, 1993.
Fick J, Barker FG, 2nd, Dazin P, et al: The extent of hetero-cellular communication mediated by gap junctions is predictive of bystander tumor cytotoxicity in vitro. Proc Natl Acad Sci USA 92:11071–11075, 1995.
Gagandeep S, Brew R, Green B, et al: Prodrug-activated gene therapy: involvement of an immunological component in the “bystander effect”. Cancer Gene Ther 3:83–88, 1996.
Mullen CA, Kilstrup M and Blaese RM: Transfer of the bacterial gene for cytosine deaminase to mammalian cells conferslethal sensitivity to 5-fluorocytosine: a negative selection system. Proc Natl Acad Sci USA 89:33–37, 1992.
Huber BE, Austin EA, Good SS, et al: In vivo antitumor activity of 5-fluorocytosine on human colorectal carcinoma cells genetically modified to express cytosine deaminase. Cancer Res 53:4619–4626, 1993.
Huber BE, Austin EA, Richards CA, et al: Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci USA 91:8302–8306, 1994.
Hoganson DK, Batra RK, Olsen JC and Boucher RC: Comparison of the effects of three different toxin genes and their levels of expression on cell growth and bystander effect in lung adenocarcinoma. Cancer Res 56:1315–1323, 1996.
Connors TA: The choice of prodrugs for gene directed enzyme prodrug therapy of cancer. Gene Therapy 2:1–9, 1995.
Wei MX, Tamiya T, Chase M, et al: Experimental tumor therapy in mice using the cyclophosphamide-activating cytochrome P450 2B1 gene. Hum Gene Ther 5:969–978, 1994.
Chen L and Waxman DJ: Intratumoral activation and enhanced chemotherapeutic effect of oxazaphosphorines following cytochrome P-450 gene transfer: development of a combined chemotherapy/cancer gene therapy strategy. Cancer Res 55:581–589, 1995.
Manome Y, Wen PY, Chen L, et al: Gene therapy for malignant gliomas using replication incompetent retroviral and adenoviral vectors encoding the cytochrome P450 2B1 gene together with cyclophosphamide. Gene Ther 3:513–520, 1996.
Chen L, Waxman DJ, Chen D and Kufe DW: Sensitization of human breast cancer cells to cyclophosphamide and ifosfamide by transfer of a liver cytochrome P450 gene. Cancer Res 56:1331–1340, 1996.
Chen L, Chen D, Manome Y, et al: Breast cancer selective gene expression and therapy mediated by recombinant adenoviruses containing the DF3/MUC1 promoter. J Clin Invest 96:2775–2782, 1995.
Wei MX, Tamiya T, Rhee RJ, et al: Diffusible cytotoxic metabolites contribute to the in vitro bystander effect associated with the cyclophosphamide/cytochrome P450 2B1 cancer gene therapy paradigm. Clin Cancer Res 1:1171–1177, 1995.
Romanini A, Sobrero AF, Chou TC, et al: Enhancement of trimetrexate cytotoxicity in vitro and in vivo by carboxypeptidase G2. Cancer Res 49:6019–6023, 1989.
Marais R, Spooner RA, Light Y, et al: Gene-directed enzyme prodrug therapy with a mustard prodrug/carboxypeptidase G2 combination. Cancer Res 56:4735–4742, 1996.
Minton NP, Atkinson T, Bruton CJ and Sherwood RF: The complete nucleotide sequence of the Pseudomomas gene coding for carboxypeptidase G2. Gene 31:31–38, 1984.
Springer CJ, Spooner RA, Light Y, et al: Intracellular and extracellular expression of the carboxypeptidase G2 enzyme for activation of a mustard prodrug in gene-directed enzyme prodrug therapy (GDEPT). B J Cancer 75 (Suppl 1): 13, 1997.
Michael NP, Brehm JK, Anlezark GM and Minton NP: Physical characterisation of the Escherichia coli B gene encoding nitroreductase and its over-expression in Escherichia coli K12. Fems Microbiol Lett 124:195–202, 1994.
Anlezark GM, Melton RG, Sherwood RF, et al: Bioactivation of dinitrobenzamide mustards by an E. coli B nitroreductase. Biochem Pharmacol 50:609–618, 1995.
Knox RJ, Friedlos F, Sherwood RF, et al: The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954) - II. A comparison of an Escherichia coli nitroreductase and Walker DT diaphorase. Biochem Pharmacol 44:2297–2301, 1992.
Knox RJ, Friedlos F, Jarman M and Roberts JJ: A new cytotoxic, DNA interstrand crosslinking agent, 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide, is formed from 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by a nitroreductase enzyme in Walker carcinoma cells. Biochem Pharmacol 37:4661–4669, 1988.
Anlezark GM, Melton RG, Sherwood RF, et al: The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954) - I. Purification and properties of a nitroreductase enzyme from Escherichia coli- a potential enzyme for antibody-directed enzyme prodrug therapy (ADEPT). Biochem Pharmacol 44:2289–2295, 1992.
Knox RJ, Friedlos F, Jarman M, et al: Virtual cofactors for an Escherichia-coli nitroreductase enzyme - relevance to reductively activated prodrugs in antibody-directed enzyme prodrug therapy (ADEPT). Biochem Pharmacol 49:1641–1647, 1995.
Friedlos F and Knox RJ: Metabolism of NAD(P)H by blood components. Relevance to bioreductively activated prodrugs in a targeted enzyme therapy system. Biochem Pharmacol 44:631–635, 1992.
Bridgewater JA, Springer CJ, Knox RJ, et al: Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur J Cancer 31a: 2362–2370, 1995.
Bailey SM and Hart IR: Nitroreductase activation of CB1954—an alternative ‘suicide’ gene system. Gene Ther 4:80–81, 1997.
Bridgewater JA, Knox RJ, Pitts JD, et al: The bystander effect of the nitroreductase CB 1954 enzyme prodrug system is due to a cell-permeable metabolite. Hum Gene Ther 8:709–717, 1997.
Bailey SM, Knox RJ, Hobbs SM, et al: Investigation of alternative prodrugs for use with E. coli nitroreductase in ‘suicide gene’ approaches to cancer therapy. Gene Ther 3:1143–1150, 1996.
Friedlos F, Denny WA, Palmer BD and Springer CJ: Mustard prodrugs for activation by Escherichia coli nitroreductase in gene-directed enzyme prodrug therapy. J Med Chem 40:1270–1275, 1997.
Manger AB, Burke PJ, Somani HH, et al: Self-immolative prodrugs: candidates for antibody-directed enzyme prodrug therapy in conjunction with a nitroreductase enzyme. J Med Chem 37:3452–3458, 1994.
Hay MP, Wilson WR and Denny WA: A novel enediyne pro-drug for antibody-directed enzyme prodrug therapy (ADEPT) using E. coli B nitroreductase. Bioorg Med Chem Lett 5:2829–2834, 1995.
Tercel M, Denny WA and Wilson WR: A novel nitro-substituted seco-CI: application as a reductively activated ADEPT prodrug. Bioorg Medicinal Chem Letter 6:2741–2744, 1996.
Lee M, Simpson JE, Woo S, et al: Synthesis of an aminopropyl, analog of the experimental anticancer drug tallimustine, and activation of its 4- nitrobenzylcarbamoyl prodrug by nitroreductase and NADH. Bioorg Medicinal Chem Letter 7:1065–1070, 1997.
Patterson AV, Zhang H, Moghaddam A, et al: Increased sensitivity to the prodrug 5′-deoxy-5-fluorouridine and modulation of 5-fluoro-2′-deoxyuridine sensitivity in MCF-7 cells transfected with thymidine phosphorylase. Br J Cancer 72:669–675, 1995.
Manome Y, Wen PY, Dong Y, et al: Viral vector transduction of the human deoxycytidine kinase cDNA sensitizes glioma cells to the cytotoxic effects of cytosine arabinoside in vitro and in vivo. Nat Med 2:567–573, 1996.
Frei E, Teicher BA, Holden SA, et al: Preclinical studies and clinical correlation of the effect of alkylating dose. Cancer Res 48:6417–6423, 1988.
Fitzsimmons SA, Workman P, Grever M, et al: Reductase enzyme expression across the National Cancer Institute Tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst 88:259–269, 1996.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knox, R.J., Connors, T.A. Prodrugs in cancer chemotherapy. Pathol. Oncol. Res. 3, 309–324 (1997). https://doi.org/10.1007/BF02904292
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02904292