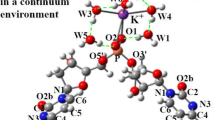
Abstract
Complexation of a phenanthridine dye ethidium bromide with a desoxyoligonucleotide 5’-d(GpApCpAp-TpGpTpC) in aqueous salt solution is studied by one- and two-dimensional1H NMR spectroscopy. Two-dimensional correlated homonuclear PMR spectroscopy (2D-TOCSY and 2D-N0ESY) was used for complete assignment of the proton signals of molecules in solution and for qualitative analysis of the character of interaction between ethidium bromide and desoxyoctanucleotide. The concentration dependences of the proton chemical shifts of the molecules were measured at three temperatures (T1 = 298 K, T2 = 308 K, and T3 = 318 K); the temperature dependences were measured in the temperature range 278–358 K. Different schemes of dye complexation with an octamer duplex involving different molecular associates in solution are considered. The equilibrium constants of the reactions, the corresponding thermodynamic parameters (δH0, δS0), and the limiting values of the chemical shifts of ethidium bromide protons in the complexes are determined. The relative contents of complexes of different types in solution (dye complexes with desoxyoctanucleotide in duplex form) are analyzed, and peculiarities of the dynamic equilibrium depending on the ratio of dye and octamer concentrations and temperature are established. The most probable structures of the 1:2 and 2:2 intercalated complexes corresponding to dye intercalation into the pyrimidine-purine sites of the desoxyoctanucleotide duplex are derived using the calculated values of the induced proton chemical shifts of ethidium bromide and two-dimensional PMR data.
Similar content being viewed by others
References
D. D. Albergo, L. A. Marky, K. J. Breslauer, and D. H. Turner,Biochemistry,20, 1409–1417 (1981).
M. Petersheim and D. H. Turner,ibid.,12, 256–263 (1983).
S. A. Bailey, D. A. Graves, R. Rill, and G. Marsh,ibid.,32, 5881–5887 (1993).
S. A. Bailey, D. A. Graves, and R. Rill,ibid.,33, 11493–11500 (1994).
A. N. Veselkov, D. B. Davies, L. N. Djimant, et al.,Biopolim. Klet., No. 7, 15–22 (1991).
A. N. Veselkov, L. N. Djimant, V. V. Kodintsev, et al.,Biofizika,40, 283–291 (1995).
D. B. Davies and A. N. Veselkov,J. Chem. Soc, Faraday Trans.,92, 3545–3557 (1996).
D. B. Davies, L. N. Djimant, S. F. Baranovsky, and A. N. Veselkov,Biopolymers,42, 285–295 (1997).
K. J. Breslauer, R. Frank, H. Blocker, and L. A. Marky,Proc. Natl. Acad. Sci. USA,83, 3746–3750 (1986).
I. Hernandez, M. Zhong, S. H. Courtney, et al.,Biochemistry,33, 13140–13152 (1994).
J. W. Nelson and I. Tinoco Jr.,Biopolymers,23, 213–224 (1984).
H. P. Hopkins, J. Fumero, and W. D. Wilson,ibid.,29, 445–459 (1990).
D. B. Davies, L. Karawajew, and A. N. Veselkov,ibid.,38, 745–757 (1996).
A. N. Veselkov, L. N. Djimant, P. A. Bolotin, et al.,Zh. Strukt. Khim.,37, 1243–1355 (1996).
J. L. Bresloff and D. M. Crothers,Biochemistry,20, 3547–3553 (1981).
D. B. Davies, L. N. Djimant, and A. N. Veselkov,J. Chem. Soc, Faraday Trans.,92, 183–390 (1996).
A. N. Veselkov, S. G. Osetrov, V. I. Pakhomov, et al.,Biopolim. Klet.,14, No. 3, 184–190 (1998).
A. N. Veselkov, L. N. Djimant, L. S. Karawajew, and E. L. Kulikov,Stud. Biophysica,106, 171–180 (1985).
D. B. Davies, S. F. Baranovsky, and A. N. Veselkov,J. Chem. Soc, Faraday Trans.,93, 1559–1572 (1997).
C. Giessner-Piettre and B. Pullman,Quart. Rev. Biophys.,20, 113–172 (1987).
V. I. Poltev and A. V. Teplukhin,Molek. Biol.,21, 102–115 (1987).
V. I. Poltev and A. V. Teplukhin,Int. J. Quant. Chem.,35, 91–102 (1989).
R. E. Dickerson,J. Biomol. Struct. Dyn.,6, 627–634 (1989).
S. C. Jain and H. M. Sobell,ibid.,1, 1179–1194 (1984).
T. Lybrand and P. Kollman,Biopolymers,24, 1863–1879 (1985).
K.-X. Chen, N. Gresh, and B. Pullman,ibid.,26, 831–848 (1987).
J. M. Sturtevant,Proc. Natl. Acad. Sci. USA,74, 2236–2240 (1977).
K. E. Reinert,Nucleic Acids Res.,11, 3411–3430 (1983).
J. B. Chaires,Biopolymers,24, 403–419 (1985).
D. Rentzeperis, M. Medero, and L. A. Marky,Bioorg. Med. Chem.,3, 751–759 (1995).
Author information
Authors and Affiliations
Additional information
Translated fromZhurnal Strukturnoi Khimii, Vol.40, No. 2, pp. 265–275, March–April, 1999.
Rights and permissions
About this article
Cite this article
Veselkov, A.N., Djimant, L.N., Pakhomov, V.I. et al. Analysis of the interaction of ethidium bromide with a DNA octamer 5’-d(GpApCpApTpGpTpC) in aqueous solution using1H NMR data. J Struct Chem 40, 220–229 (1999). https://doi.org/10.1007/BF02903650
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02903650